Lercanidipine
General Information
Lercanidipine Impurities and Lercanidipine
Daicel Pharma offers worldwide delivery options for custom synthesis of Lercanidipine impurity standards, including crucial impurity standards such as R-Lercanidipine and S-Lercanidipine. These impurity standards play a vital role in evaluating the purity and safety of Lercanidipine, an active pharmaceutical ingredient.
Raynaud’s syndrome, angina pectoris management, and hypertension are the therapeutic uses of Lercanidipine [CAS: 100427-26-7]. It is a diarylmethane compound and belongs to the dihydropyridine class of calcium channel blockers.
Lercanidipine: Use and Commercial Availability
Lercanidipine, available under Zanidip, is a lipophilic dihydropyridine calcium antagonist. It has a long receptor half-life and exhibits a slow onset of action, which helps prevent reflex tachycardia often associated with other dihydropyridines. Lercanidipine provides consistent and sustained blood pressure reduction. It demonstrates comparable antihypertensive efficacy to other agents and can be used as initial monotherapy or combined with other medications.
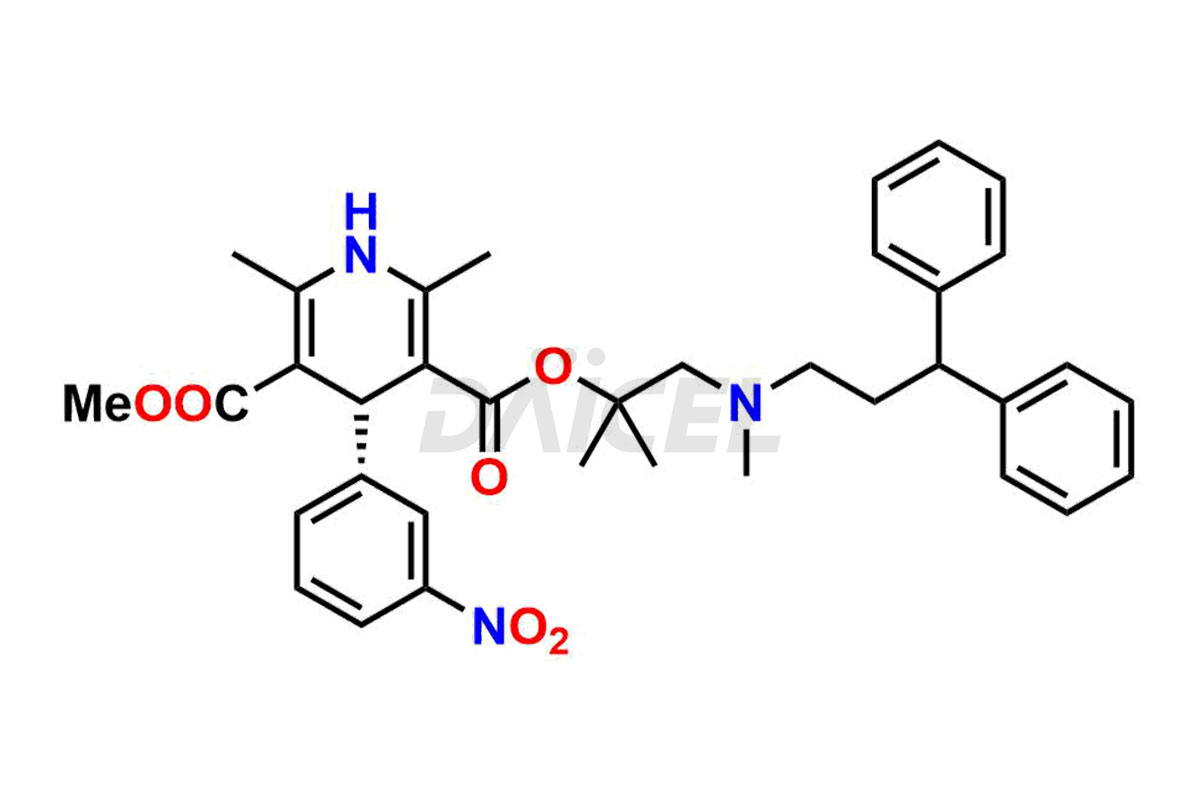
Lercanidipine Structure and Mechanism of Action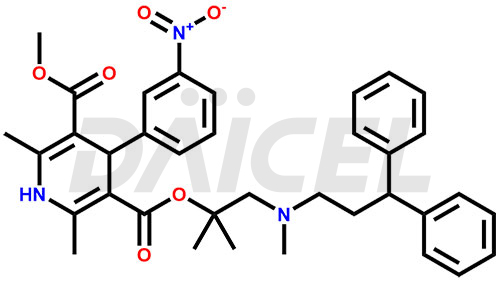
The chemical name of Lercanidipine is 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 3-[2-[(3,3-diphenylpropyl)methylamino]-1,1-dimethylethyl] 5-methyl ester 3,5-Pyridinedicarboxylic acid. Its chemical formula is C36H41N3O6, and its molecular weight is approximately 611.7 g/mol.
Lercanidipine prevents the entry of extracellular calcium across the vascular smooth muscle cell membranes.
Lercanidipine Impurities and Synthesis
Impurities can form during the manufacturing1 process of Lercanidipine as byproducts or degradation products. They may arise from the starting materials, reaction conditions, or other factors. Analytical techniques such as high-performance liquid chromatography (HPLC) help detect and quantify these impurities. Stringent control measures ensure that the impurity levels remain within acceptable limits, ensuring the purity and quality of Lercanidipine. Thorough analysis and monitoring help throughout the manufacturing process to maintain the safety and efficacy of the drug.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Lercanidipine impurity standards. We provide a range of Lercanidipine impurity standards, such as R-Lercanidipine and S-Lercanidipine. Our impurity standards have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Lercanidipine impurity standards or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Nardi, Dante; Leonardi, Amedeo; Graziani, Gabriele; Bianchi, Giorgio, Asymmetrical Diesters Of 1,4-Dihydro-2,6-Dimethyl-Pyridine-3,5-Dicarboxylic Acid, Recordati S. A. Chemical and Pharmaceutical Co., Switzerland, EP153016B1, May 16, 1990
- Alvarez-Lueje, A.; Pujol, S.; Squella, J. A.; Nunez-Vergara, L. J., A selective HPLC method for determination of lercanidipine in tablets, Journal of Pharmaceutical and Biomedical Analysis, Volume: 31, Issue: 1,Pages: 1-9, 2003
Frequently Asked Questions
What steps are taken to identify and characterize Lercanidipine impurities?
Extensive analytical testing is conducted to identify and characterize impurities in Lercanidipine. Techniques such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy help in impurity profiling.
Are Lercanidipine impurities monitored throughout its shelf life?
Lercanidipine impurities are monitored throughout their shelf life to ensure their levels remain within acceptable limits. Stability studies help assess their degradation and formation over time.
Can Lercanidipine impurities be removed or reduced through purification processes?
Yes, purification processes during the manufacturing of Lercanidipine can help remove or reduce impurities. Techniques like recrystallization, filtration, or chromatography help improve the purity of the drug substance.
How should Lercanidipine impurities be stored in terms of temperature?
The recommendation is to store Lercanidipine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.