LOAD MORE
You're viewed 9 of 27 products
Daicel Pharma offers worldwide delivery options for custom synthesis of Lenvatinib impurity standards, including crucial impurity standards such as 2,4-dichloro-7-methoxyquinoline-6-carboxamide, 3,4-dichloro-7-methoxyquinoline-6-carboxamide, 4-(4-amino-3-hydroxyphenoxy)-7-methoxyquinoline-6-carboxamide, 4-Amino-2,3,5-Trichlorophenol, Lenvatinib chloro impurity, Lenvatinib Impurity-10 and more. These impurity standards play a vital role in evaluating the purity and safety of Lenvatinib, an active pharmaceutical ingredient.
Lenvatinib [CAS: 417716-92-8] is an orally available receptor tyrosine kinase inhibitor and antineoplastic agent. It treats refractory renal cell carcinoma and advanced, metastatic medullary thyroid cancer.
Lenvatinib treats unresectable or advanced hepatocellular carcinoma (HCC), radioactive iodine-refractory differentiated thyroid cancer (DTC), and advanced renal cell carcinoma (RCC). Marketed under the brand name Lenvima, it is a multiple receptor tyrosine kinase inhibitor that effectively inhibits VEGF receptors, leading to potent antiangiogenic effects. Furthermore, Lenvatinib blocks FGFR, RET, PDGFRa, and KIT, causing the suppression of malignant cell proliferation.
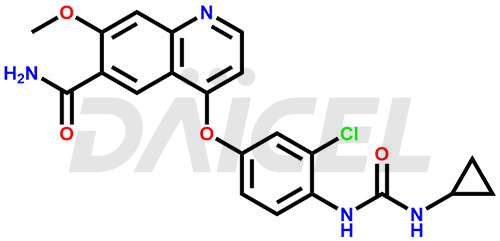
The chemical name of Lenvatinib is 4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide. Its chemical formula is C21H19ClN4O4, and its molecular weight is approximately 426.9 g/mol.
Lenvatinib blocks the kinase activities of vascular endothelial growth factor (VEGF) receptors and fibroblast growth factor (FGF) receptors, FGFR1, 2, 3, and 4.
During the production1 of Lenvatinib, impurities can form as byproducts or degradation products. They may arise from the starting materials, intermediates, or reaction conditions. It is crucial to identify, characterize, and control these impurities to ensure the safety and efficacy of the drug. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help detect and quantify impurities. Strict quality control measures ensure impurity levels remain within acceptable limits, thereby maintaining the purity and quality of Lenvatinib.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Lenvatinib impurity standards. We provide a range of Lenvatinib impurity standards, such as 2,4-dichloro-7-methoxyquinoline-6-carboxamide, 3,4-dichloro-7-methoxyquinoline-6-carboxamide, 4-(4-amino-3-hydroxyphenoxy)-7-methoxyquinoline-6-carboxamide, 4-Amino-2,3,5-Trichlorophenol, Lenvatinib chloro impurity, Lenvatinib Impurity-10 and more. Our impurity standards have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Lenvatinib impurity standards or degradation products. Each delivery has a comprehensive characterization report.
Impurities in Lenvatinib can form during the manufacturing process, storage conditions, or due to interactions with other components. Factors such as temperature, pH, and exposure to light can contribute to impurity formation.
Regulatory authorities, such as the United States Pharmacopeia (USP) or the International Council for Harmonization (ICH), provide guidelines on acceptable limits for impurities in Lenvatinib to ensure its safety and quality.
Impurities in Lenvatinib have the potential to impact its efficacy or safety. Some may have toxic effects or alter the drug's therapeutic properties, making it crucial to control them within acceptable limits.
The recommendation is to store Lenvatinib impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.