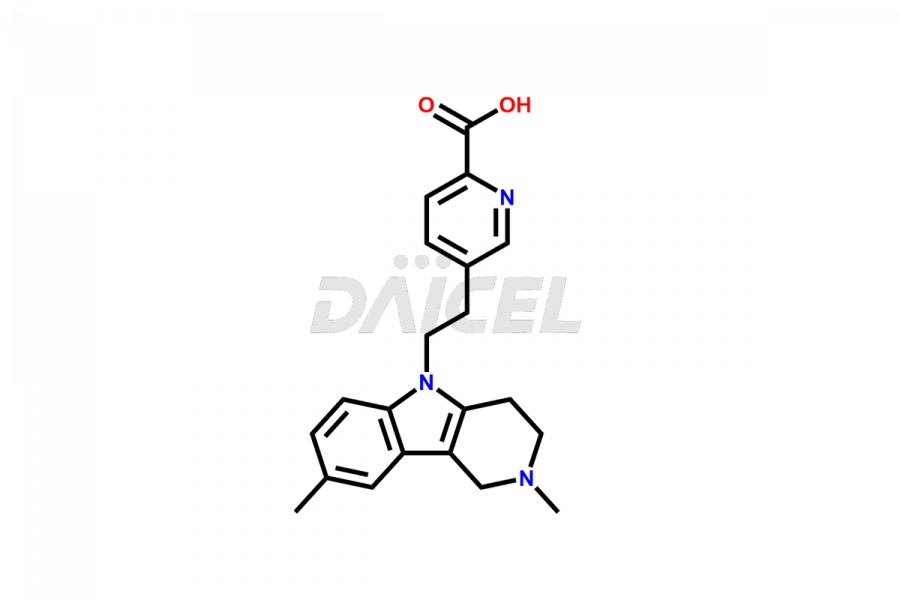
Latrepirdine
General Information
Latrepirdine Impurities and Latrepirdine
Daicel Pharma offers worldwide delivery options for custom synthesis of Latrepirdine impurities, including impurity standards, such as Latrepirdine Impurity 2, Latrepirdine Impurity-1, and Latrepirdine N-oxide. These impurities play a vital role in evaluating the purity and safety of Latrepirdine, an active pharmaceutical ingredient.
Investigated for its potential therapeutic benefits, Latrepirdine [CAS: 3613-73-8] is a compound belonging to the methylpyridines and pyridoindole class. It treats Alzheimer’s Disease and Huntington’s Disease.
Latrepirdine: Use and Commercial Availability
Latrepirdine was initially marketed as a non-selective antihistamine in Russia and later repurposed as a treatment for Alzheimer’s disease (AD) and Huntington’s disease (HD). However, due to its wide range of neuronal functions, exploring the synthesis of structurally similar compounds and investigating their effects on therapeutic targets could lead to novel dementia treatments.
Latrepirdine Structure and Mechanism of Action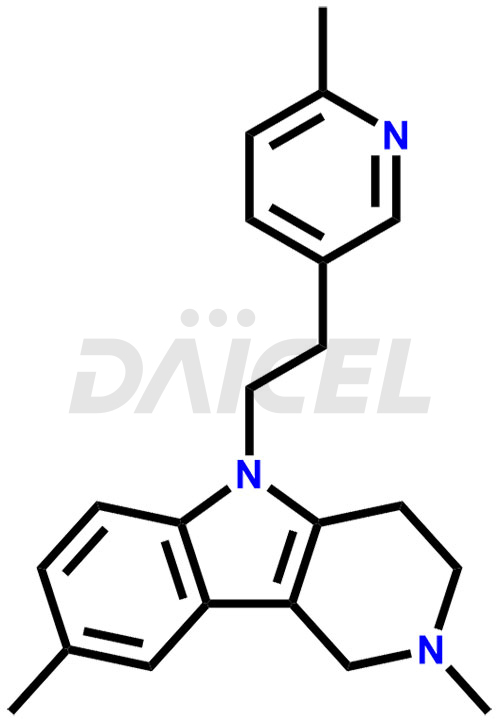
The chemical name of Latrepirdine is 2,3,4,5-Tetrahydro-2,8-dimethyl-5-[2-(6-methyl-3-pyridinyl)ethyl]-1H-pyrido[4,3-b]indole. Its chemical formula is C21H25N3, and its molecular weight is approximately 319.4 g/mol.
Latrepirdine blocks H1 histamine receptor activity. It also affects coronary blood flow and myocardial contractibility. The drug’s exact mechanisms of action and how it contributes to clinical benefits remain uncertain.
Latrepirdine Impurities and Synthesis
Latrepirdine is a medication that treats Alzheimer’s disease. As with any pharmaceutical compound, Latrepirdine can contain impurities. Impurities in latrepirdine can arise from the manufacturing process1 or external factors. Common Latrepirdine impurities in formulations include related compounds, such as isomers or degradation products. They can result from synthesis, storage conditions, or interactions with other substances. It is crucial for pharmaceutical manufacturers to carefully monitor and control the levels of impurities in latrepirdine to ensure its safety and efficacy. Strict quality control measures help minimize them and maintain the highest pharmaceutical standards for Latrepirdine.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Latrepirdine impurity standards. We provide a range of Latrepirdine impurity standards, such as Latrepirdine Impurity 2, Latrepirdine Impurity-1, and Latrepirdine N-oxide. Our impurities have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis. We give additional data, such as 13C-DEPT, upon request. We can synthesize unknown Latrepirdine impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
Frequently Asked Questions
What measures can control Latrepirdine impurities during packaging?
Appropriate packaging materials protect Latrepirdine from exposure to light, moisture, and other environmental factors and can help control impurities during packaging.
Can Latrepirdine impurities vary based on the manufacturing process used?
The choice of the manufacturing process can influence the impurity profile of Latrepirdine. Different synthetic routes or variations in reaction conditions can lead to varying impurity profiles.
Are there specific methods for removing Latrepirdine impurities from the drug during purification?
Purification techniques such as recrystallization, column chromatography, filtration, and extraction can remove impurities from Latrepirdine.
How should Latrepirdine impurities be stored in terms of temperature?
The recommendation is to store Latrepirdine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.