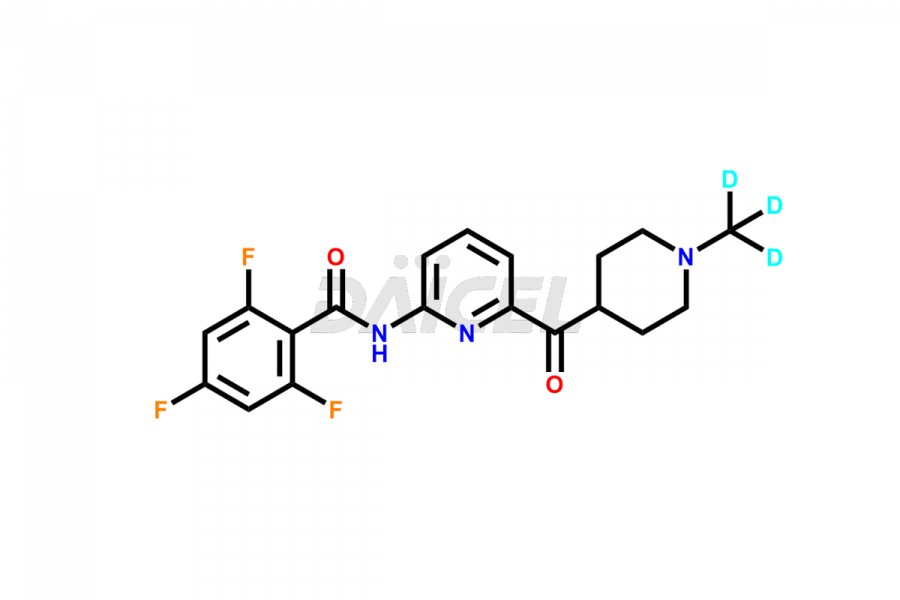
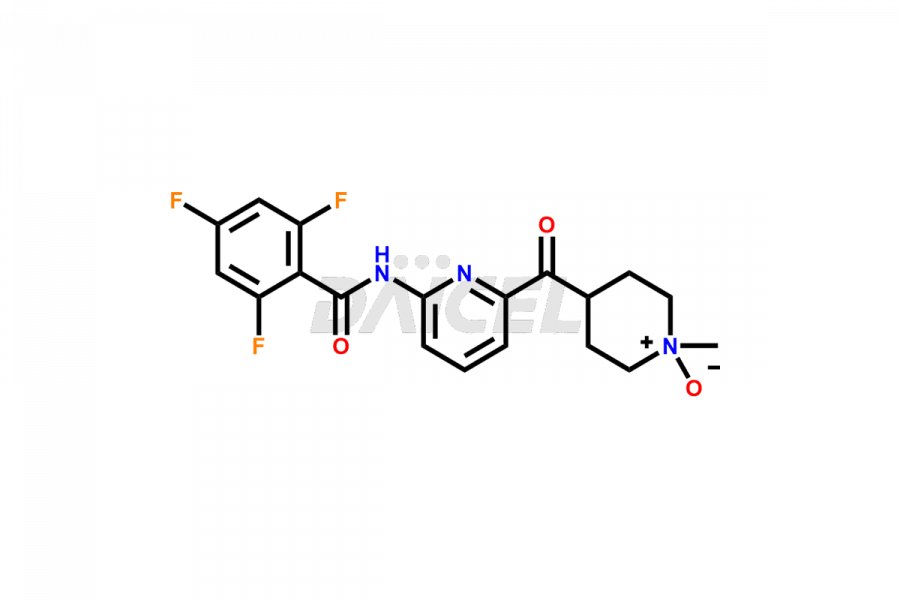
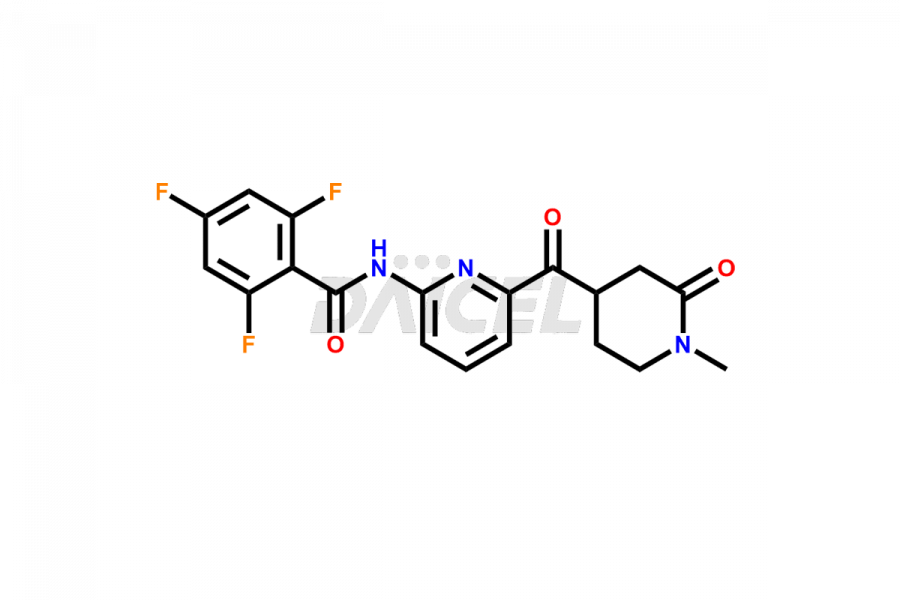
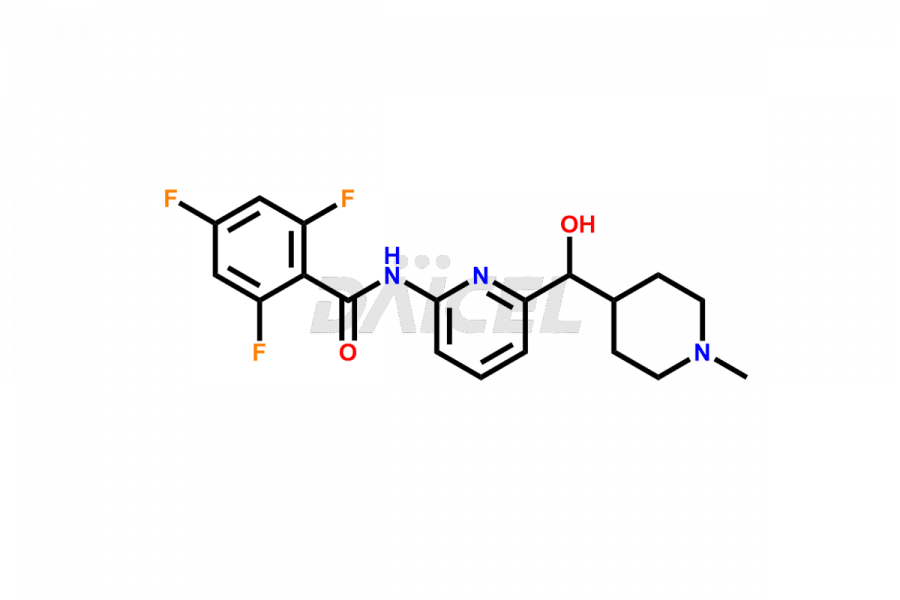
Lasmiditan
General Information
Lasmiditan Impurities and Lasmiditan
Daicel Pharma offers worldwide delivery options for a custom synthesis of Lasmiditan impurities, including impurities such as the M3 Metabolite of Lasmiditan, M7 Metabolite of Lasmiditan, and M8 Metabolite of Lasmiditan. These impurities play a vital role in evaluating the purity and safety of Lasmiditan, an active pharmaceutical ingredient.
Associated with migraine headaches, Lasmiditan [CAS: 439239-90-4] is a small molecule that selectively activates the serotonin 1F receptor (5HT1F receptor). It reduces neuron activity and contributes to the pain and inflammation experienced during migraines.
Lasmiditan: Use and Commercial Availability
In adults, Reyvow (Lasmiditan) treats migraine attacks, both with and without aura. This medication acts as an effective agonist for the serotonin 5-HT1F receptor, providing relief during the headache phase of migraines. Unlike triptans, Rayvow does not have vasoconstrictor effects, making it a suitable alternative for individuals with cardiovascular disease.
Lasmiditan Structure and Mechanism of Action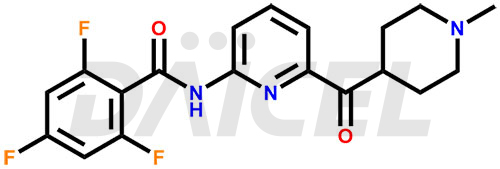
The chemical name of Lasmiditan is 2,4,6-Trifluoro-N-[6-[(1-methyl-4-piperidinyl)carbonyl]-2-pyridinyl]benzamide. Its chemical formula is C19H18F3N3O2, and its molecular weight is approximately 377.4 g/mol.
Lasmiditan treats migraine as it binds with the 5-HT1F receptor, and its mechanism of action is unknown.
Lasmiditan Impurities and Synthesis
Lasmiditan is a medication used for the acute treatment of migraine headaches. Like other pharmaceutical products, Lasmiditan can contain impurities. They can originate from various sources, including the manufacturing process1 or external factors. Common Lasmiditan impurities in formulations include related compounds, such as isomers or degradation products. Impurities can result from synthesis, storage conditions, or interactions with other substances. Manufacturers employ rigorous quality control measures to monitor and control the impurity levels of Lasmiditan, ensuring its safety and efficacy. These measures aim to minimize impurities and maintain high pharmaceutical standards for the production of Lasmiditan.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Lasmiditan impurity standards such as the M3 Metabolite of Lasmiditan, M7 Metabolite of Lasmiditan, and M8 Metabolite of Lasmiditan. We offer deuterium-labeled Lasmiditan standard, Lasmiditan-D3, essential for bioanalytical research and BA/BE studies. Our impurities have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Lasmiditan impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Cohen, Michael Philip; Kohlman, Daniel Timothy; Liang, Sidney Xi; Mancuso, Vincent; Victor, Frantz; Xu, Yao-Chang; Ying, Bai-Ping; Zacherl, Deanna Piatt; Zhang, Deyi, Pyridinoylpiperidines As 5-HT1F Agonists, Eli Lilly and Company, United States, EP1492786B1, October 4, 2006
- Solanki, Harshali; Agrawal, Ritesh; Karole, Sarita; Loksh, Kavita R., HPLC method development and validation for the estimation of lasmiditan in marketed formulation, World Journal of Pharmaceutical Research, Volume: 11, Issue: 2, Pages: 2022-2032, 2022
Frequently Asked Questions
Can Lasmiditan impurities affect its stability?
Yes, impurities in Lasmiditan can impact its stability. Some impurities may promote degradation or interact with the active ingredient leading to reduced shelf life or altered potency.
How is the safety of Lasmiditan impurities assessed?
The safety of Lasmiditan impurities is assessed through toxicological studies to determine their potential risks and establish permissible levels based on clinical data and regulatory guidelines.
Which solvent helps analyze Lasmiditan impurities?
Methanol or Acetonitrile is commonly used as a solvent when analyzing many impurities in Lasmiditan.
How should Lasmiditan impurities be stored in terms of temperature?
The recommendation is to store Lasmiditan impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.