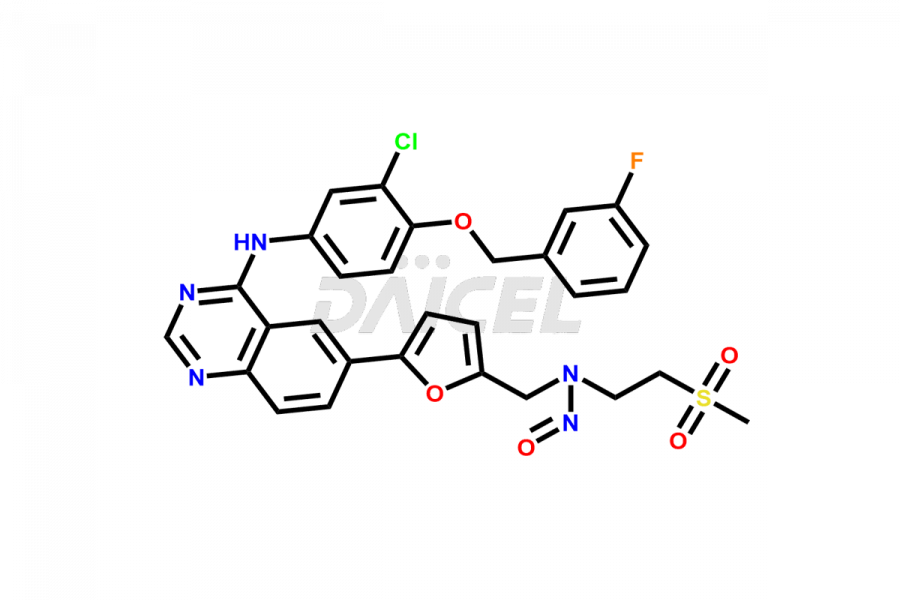
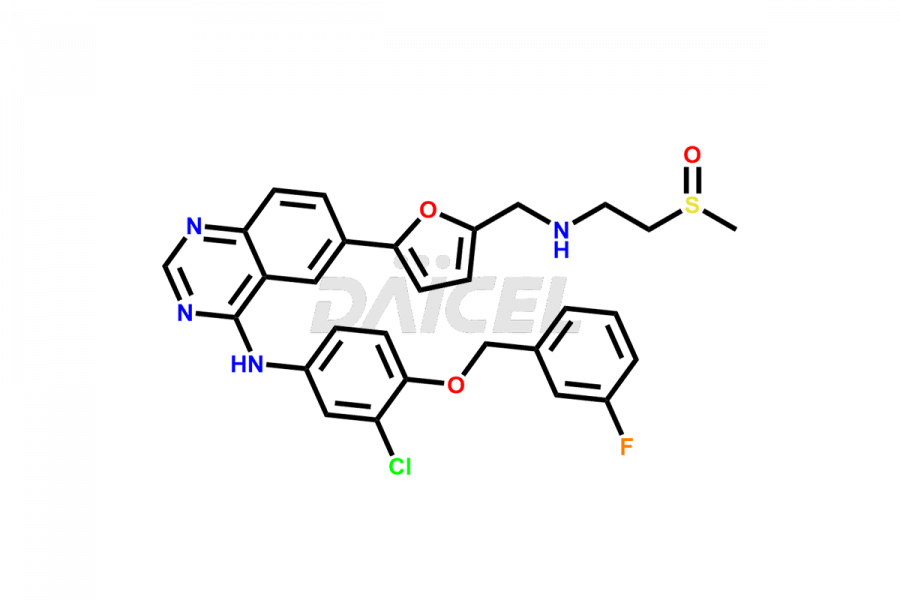
Lapatinib
References
Frequently Asked Questions
Can Lapatinib impurities be controlled through formulation adjustments?
Yes, formulation adjustments can minimize impurities in Lapatinib, such as optimizing the pH, solvent selection, or using stabilizing agents.
How are Lapatinib impurities controlled during manufacturing?
Lapatinib impurities are controlled during manufacturing through strict quality control measures, such as process optimization, purification techniques, and adherence to good manufacturing practices (GMP).
How are Lapatinib impurities analyzed?
Lapatinib impurities are analyzed using various analytical techniques, such as high-performance liquid chromatography (HPLC) or liquid chromatography (LC).
How should Lapatinib impurities be stored in terms of temperature?
The recommendation is to store Lapatinib impurities at controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.