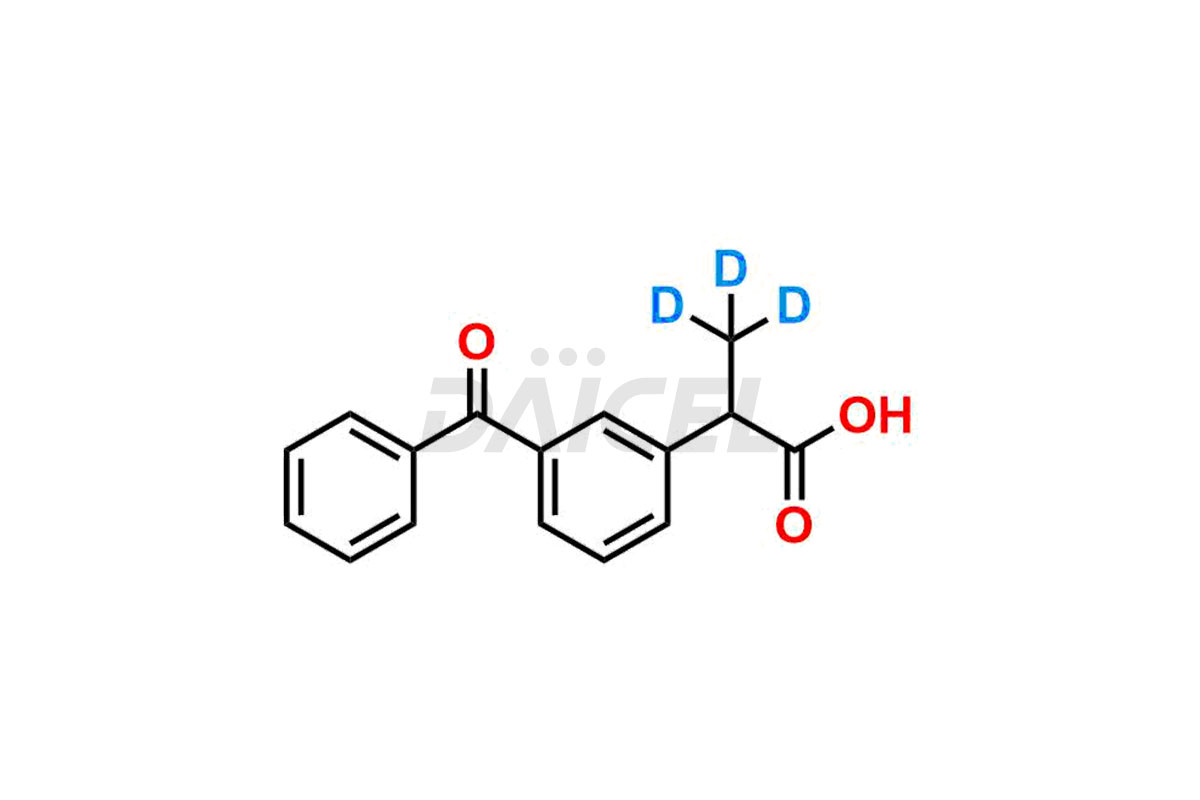
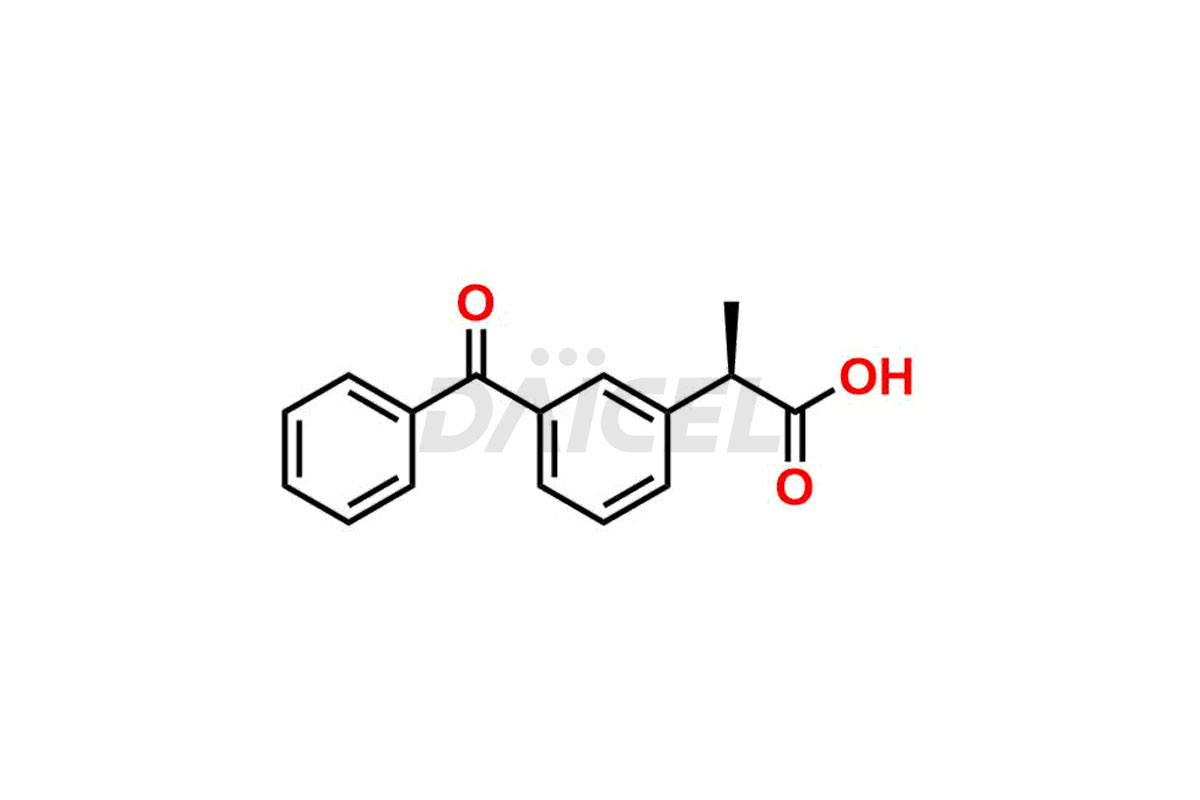
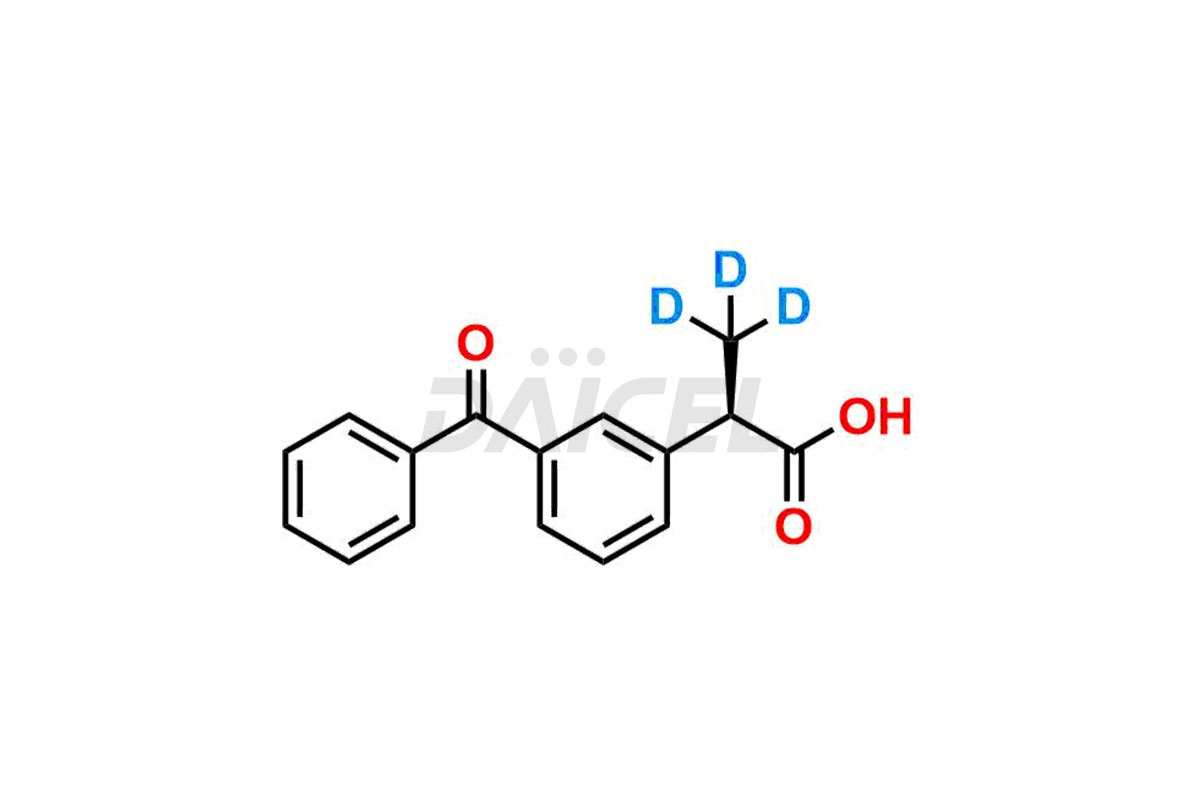
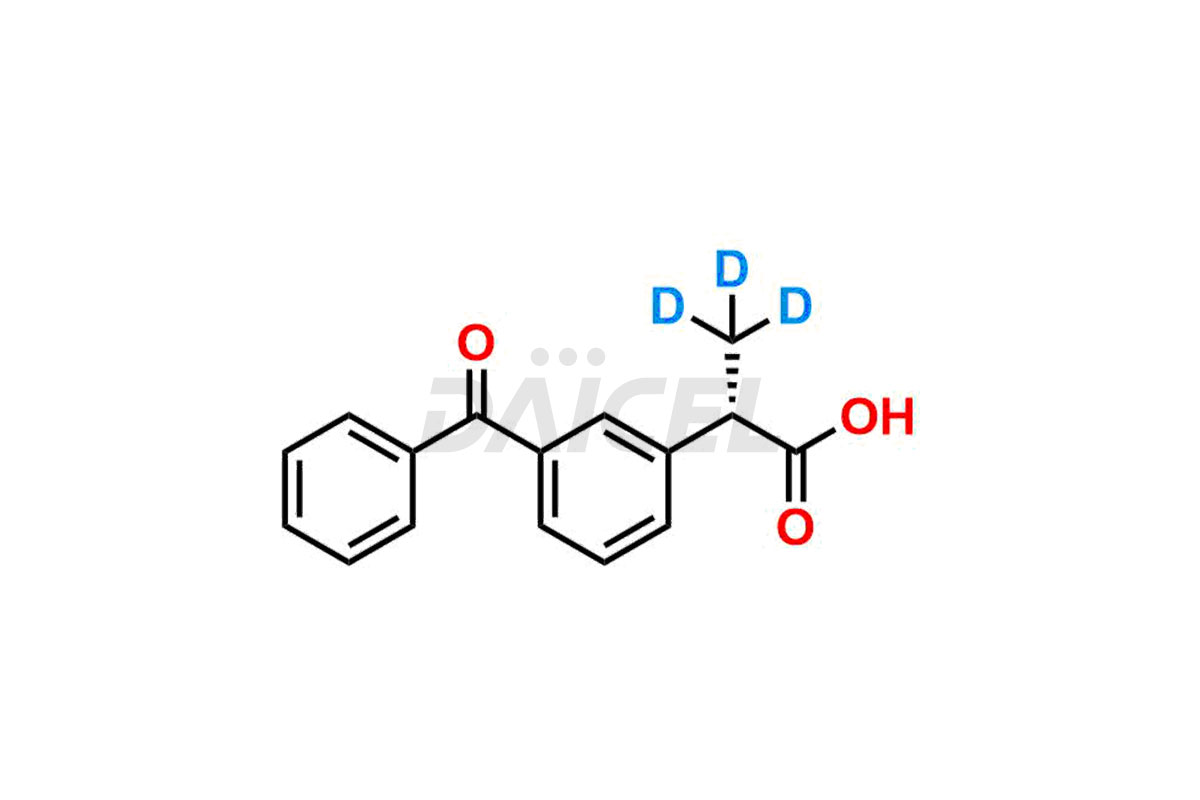
Ketoprofen
General Information
Ketoprofen Impurities and Ketoprofen
Daicel Pharma offers worldwide delivery options for a custom synthesis of Ketoprofen impurities, including Ketoprofen-R-Isomer, and Ketoprofen-S-Isomer. These impurities play a vital role in evaluating the purity and safety of Ketoprofen, an active pharmaceutical ingredient.
Ketoprofen [CAS: 22071-15-4] is a propionic acid derivative and a nonsteroidal anti-inflammatory drug (NSAID) possessing anti-inflammatory, analgesic, and antipyretic properties. It is the same class as ibuprofen, another anti-inflammatory analgesic and antipyretic medication. Ketoprofen treats rheumatoid arthritis and osteoarthritis.
Ketoprofen: Use and Commercial Availability
Ketoprofen, available under brand names such as Actron, Nexcede, Orudis, and Oruvail, is a nonsteroidal anti-inflammatory drug (NSAID) for treating acute pain and chronic arthritis. It exerts its effects by inhibiting cyclo-oxygenase I and II enzymes, which reduces the formation of prostaglandin and thromboxane precursors. It decreases prostaglandin synthesis, providing therapeutic benefits such as anti-inflammatory, analgesic, and antipyretic effects.
Ketoprofen Structure and Mechanism of Action 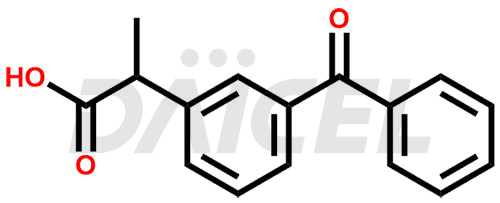
The chemical name of Ketoprofen is 3-Benzoyl-α-methylbenzeneacetic acid. Its chemical formula is C16H14O3, and its molecular weight is approximately 254.28 g/mol.
Ketoprofen inhibits prostaglandin and leukotriene synthesis and has anti bradykinin activity. Additionally, Ketoprofen inhibits platelet aggregation by suppressing thromboxane A2 synthesis.
Ketoprofen Impurities and Synthesis
Impurities, including related substances, residual solvents, and degradation products, can originate in Ketoprofen, a nonsteroidal anti-inflammatory drug (NSAID). These impurities may form during the manufacturing process1, starting materials, intermediates, or storage conditions. Strict control and monitoring of these impurities help ensure the medication’s safety and effectiveness. Regulatory authorities like the FDA have established guidelines and impurity levels in pharmaceuticals, including Ketoprofen. Manufacturers comply with these regulations and conduct comprehensive testing to identify and quantify impurities, ensuring they fall within acceptable limits.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Ketoprofen impurity standards such as Ketoprofen-R-Isomer, and Ketoprofen-S-Isomer. We offer deuterium-labeled Ketoprofen compounds, Ketoprofen-D3, Ketoprofen-R-Isomer-D3, and Ketoprofen-S-Isomer-D3 essential for bioanalytical research and BA/BE studies. Our impurities have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Ketoprofen impurities, degradation products, and labeled compounds to assess the effectiveness of generic Ketoprofen. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Farge, Daniel; Messer, Mayer N.; Moutonnier, (3-Benzoylphenyl) Alkanoic Acids, Claude, Rhone-Poulenc S. A, GB1164585A, September 17, 1969
- Jefferies, T. M.; Thomas, W. O. A.; Parfitt, R. T., Determination of ketoprofen in plasma and urine by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 162, Issue: 1, Pages: 122-4, 1979
Frequently Asked Questions
Can impurity levels in Ketoprofen vary between different manufacturers?
Ketoprofen impurity levels can vary between manufacturers as each manufacturer uses processes or starting materials, leading to differences in impurity profiles. Regulatory standards ensure that all versions meet acceptable quality criteria.
Can Ketoprofen impurities undergo transformation or interconversion over time?
Yes, impurities in Ketoprofen can undergo transformation or interconversion, particularly under certain storage conditions. It can result in the formation of new impurities or changes in the impurity profile of the medication.
Which solvent help in analyzing Ketoprofen impurities?
Methanol or Acetonitrile is commonly used as a solvent when analyzing many impurities in Ketoprofen.
How should Ketoprofen impurities be stored in terms of temperature?
The recommendation is to store Ketoprofen impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.