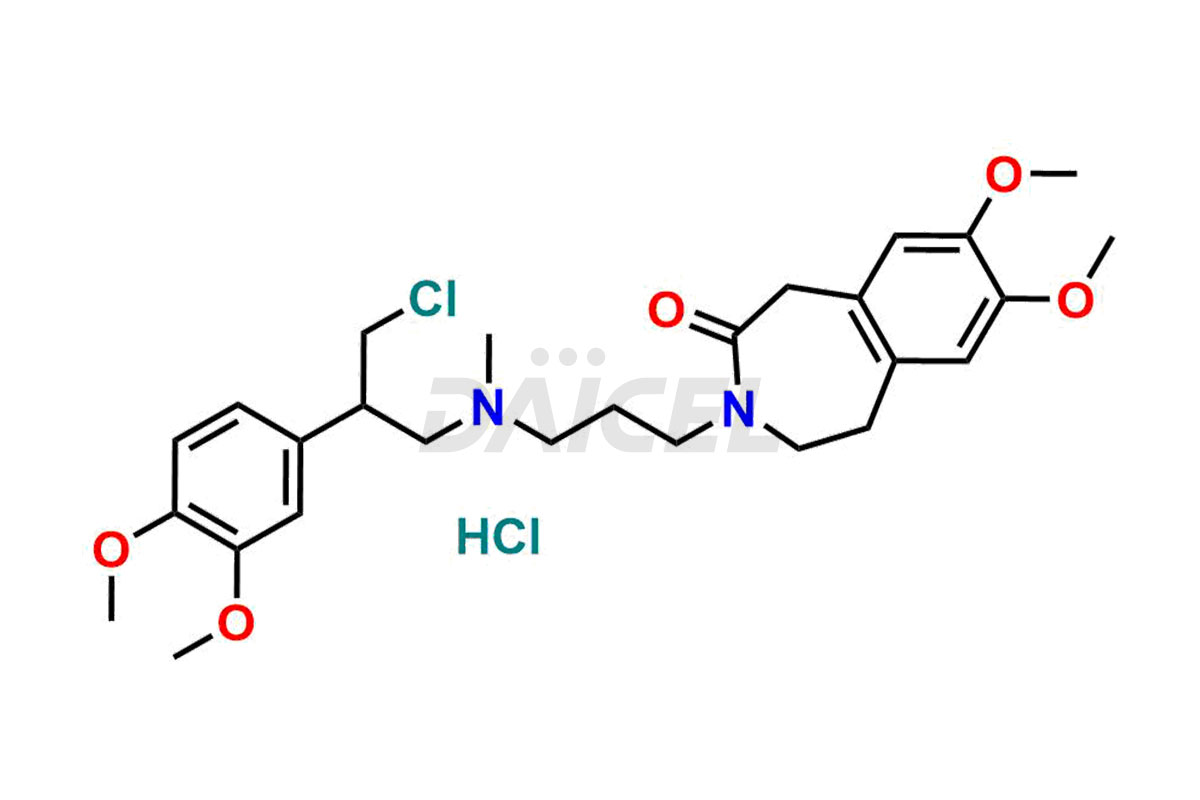
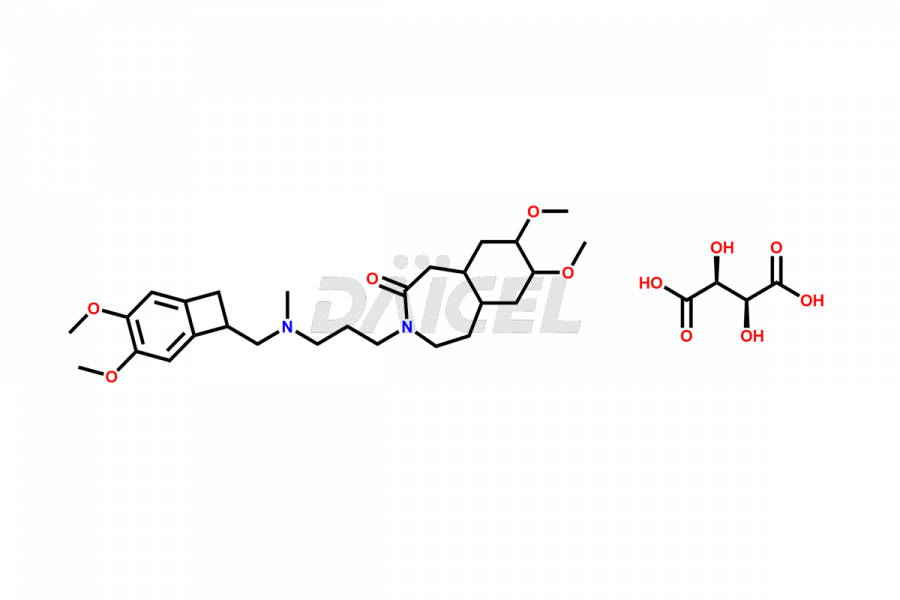
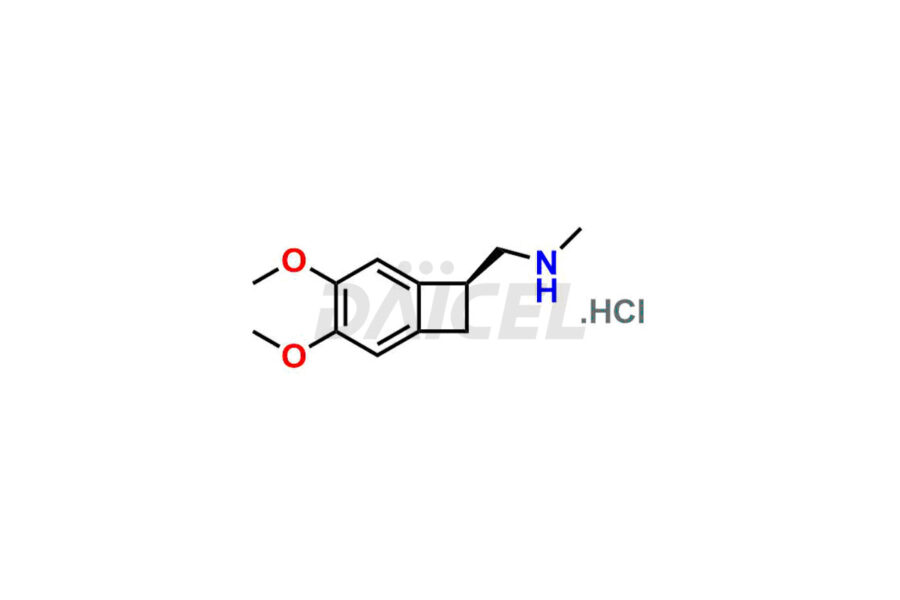
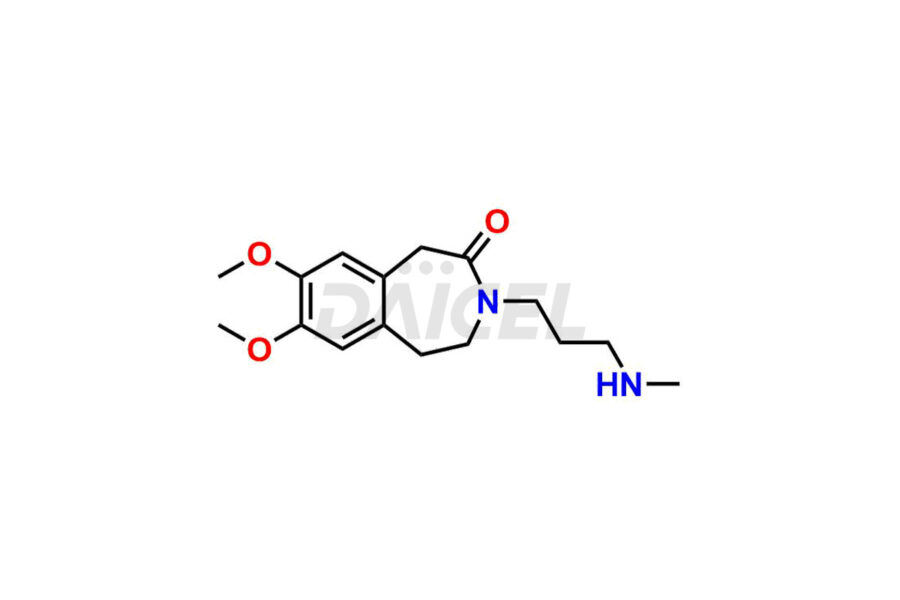
Ivabradine
General Information
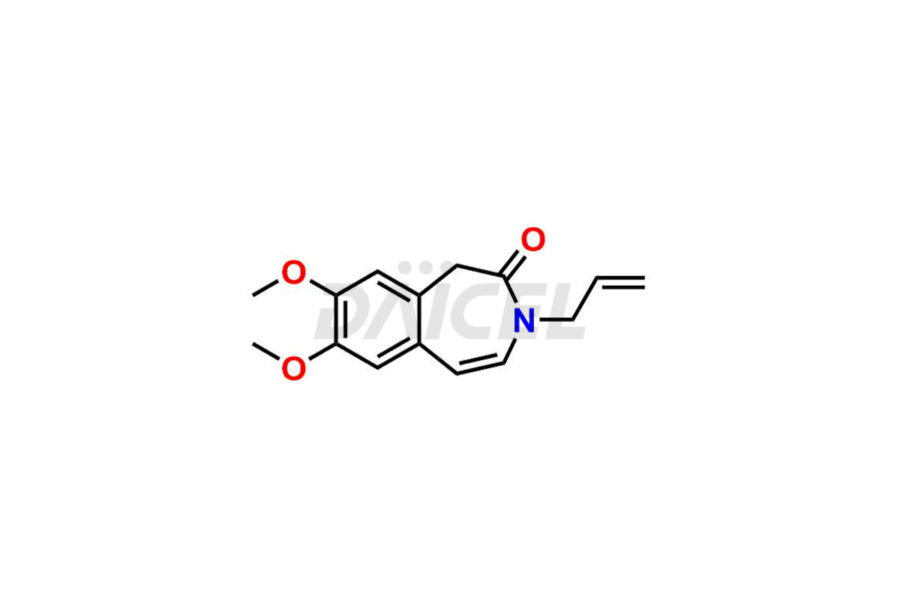
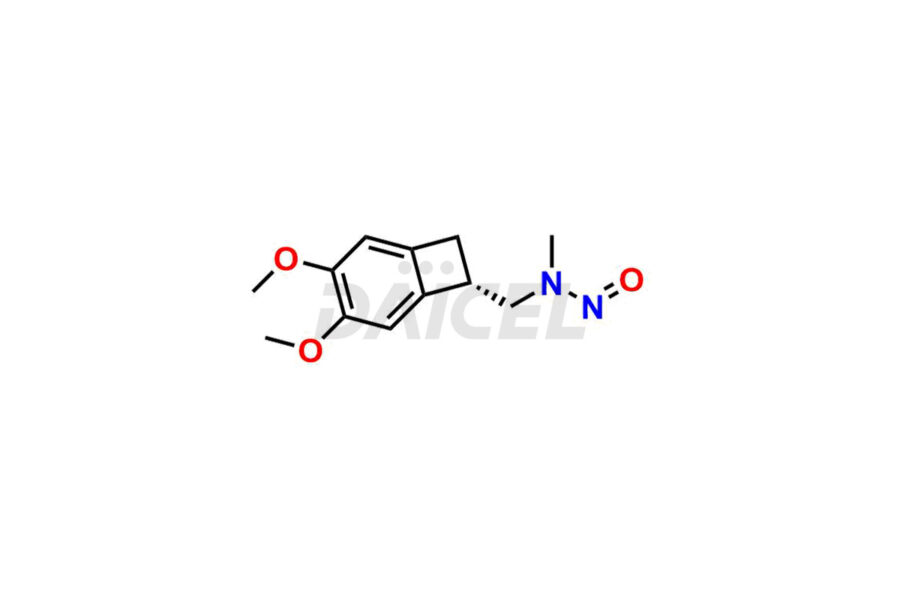
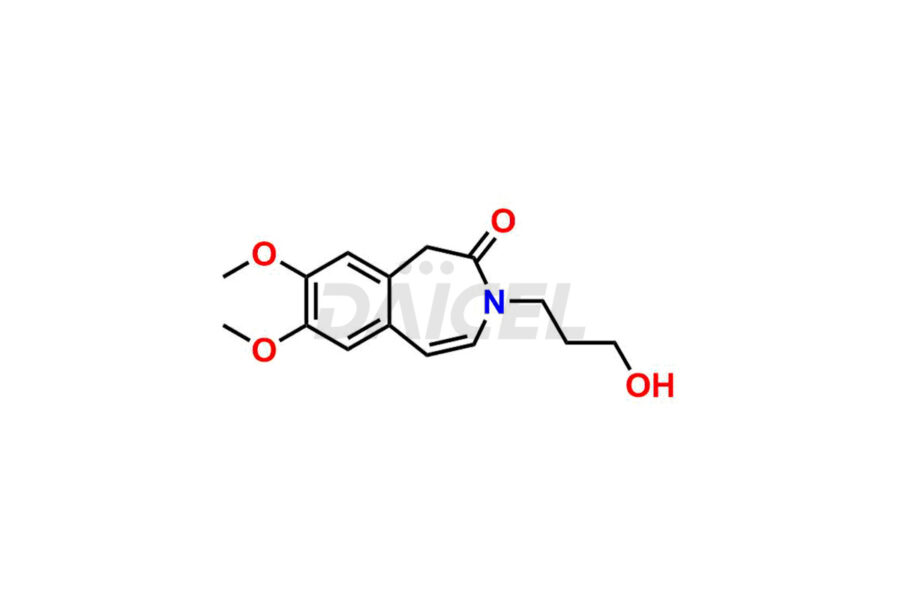
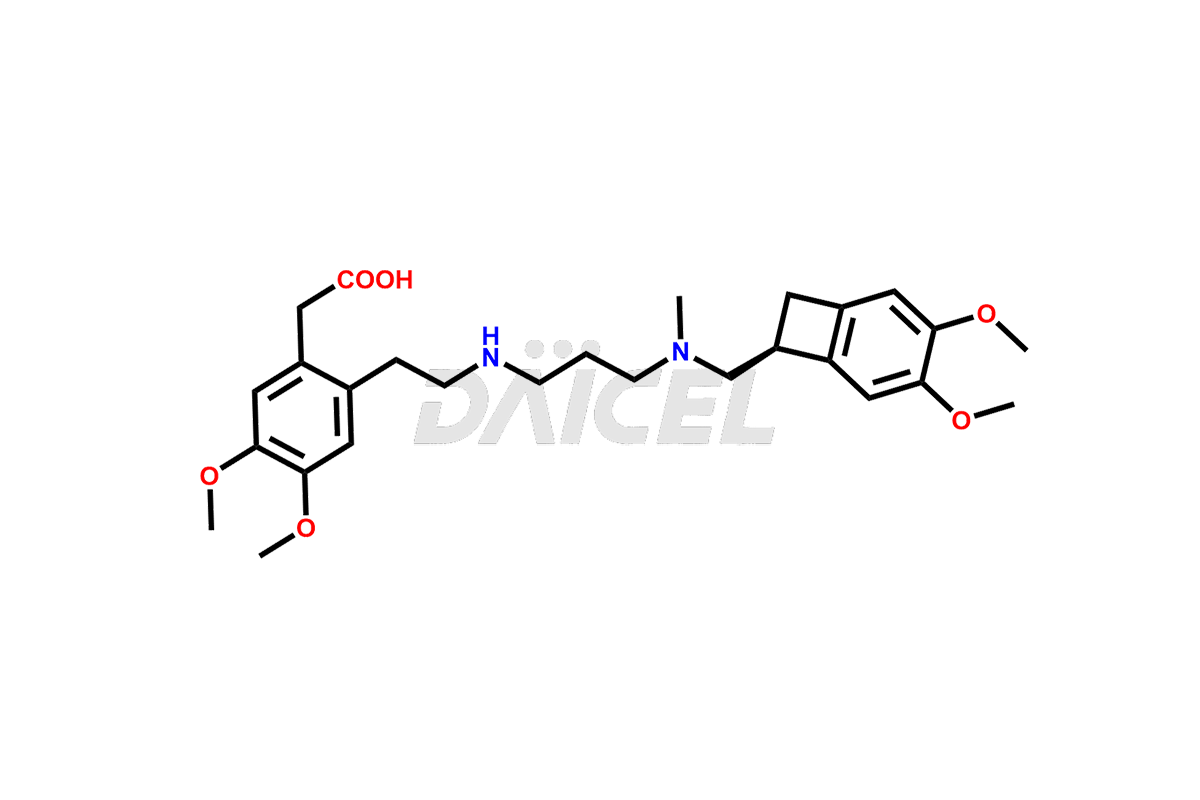
Ivabradine Impurities and Ivabradine
Daicel Pharma offers worldwide delivery options for a custom synthesis of Ivabradine impurity standards, including crucial impurity standards such as Ivabradine chloro impurity, Ivabradine impurity, Ivabradine Impurity B, Ivabradine Impurity D, Ivabradine Intermediate, Ivabradine intermediate allyl impurity, Ivabradine intermediate nitroso impurity, and Ivabradine intermediate propanol impurity. These impurity standards evaluate the purity and safety of Ivabradine, an active pharmaceutical ingredient.
Ivabradine [CAS: 155974-00-8] is a selective inhibitor of HCN channels and is used to lower the heart rate. It manages chronic stable angina when beta-adrenergic blockers are contraindicated and in treating heart failure.
Ivabradine: Use and Commercial Availability
Ivabradine, available as Corlanor, has gained FDA approval in the US for managing stable heart failure in patients. It decreases the likelihood of hospitalization caused by worsening heart failure. In Europe, Ivabradine is for treating heart failure and chronic stable angina.
Ivabradine Structure and Mechanism of Action 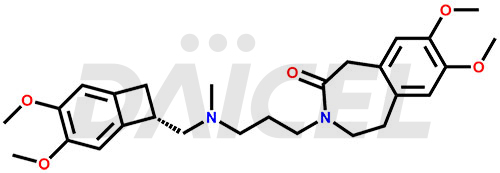
The chemical name of Ivabradine is 3-[3-[[[(7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl]methylamino]propyl]-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one. Its chemical formula is C27H36N2O5, and its molecular weight is approximately 468.6 g/mol.
Ivabradine prevents hyperpolarization-activated cyclic nucleotide-gated (HCN) channels causing the cardiac pacemaker If current regulating heart rate. It also inhibits the retinal current, Ih responsible for curtailing retinal responses to bright light stimuli.
Ivabradine Impurities and Synthesis
Ivabradine, a medication used for treating stable heart failure, can potentially contain impurities, although implementing stringent quality control measures can minimize their presence. Ivabradine impurities can originate from various sources such as during preparation1, starting materials, intermediates, or degradation products. The common Ivabradine impurities include related substances, residual solvents, and degradation products. They may arise from incomplete reactions, impure starting materials, or storage conditions. It is crucial to control and monitor these impurities to ensure the safety and efficacy of the medication. Regulatory authorities, such as the United States Food and Drug Administration (FDA), have established guidelines and impurity limits in pharmaceuticals, including Ivabradine. Manufacturers comply with these regulations and conduct thorough testing to identify and quantify impurities within acceptable limits.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Ivabradine impurity standards. We provide a range of Ivabradine impurity standards, such as Ivabradine chloro impurity, Ivabradine impurity, Ivabradine Impurity B, Ivabradine Impurity D, Ivabradine Intermediate, Ivabradine intermediate allyl impurity, Ivabradine intermediate nitroso impurity, and Ivabradine intermediate propanol impurity. Ivabradine intermediate nitroso impurity evaluates genotoxicity of Ivabradine. Our impurity standards have a detailed Certificate of Analysis (CoA) and a comprehensive characterization report. The CoA encompasses data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Additional data, such as 13C-DEPT, can be provided upon request. We can synthesize unknown Ivabradine impurity standards, degradation products, and labeled compounds to evaluate the efficacy of generic Ivabradine. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Peglion, Jean Louis; Vian, Joel; Vilaine, Jean Paul; Villeneuve, Nicole; Janiak, Philip; Bidouard, Jean Pierre, (Benzocycloalkyl) alkylamines, ADIR et Cie., France, US5296482A, March 22, 1994
- Francois-Bouchard, M.; Simonin, G.; Bossant, M.-J.; Boursier-Neyret, C., Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 745, Issue: 2, Pages: 261-269, 2000
Frequently Asked Questions
Can Ivabradine impurities impact the drug's bioavailability or pharmacokinetics?
Some impurities in Ivabradine can affect its bioavailability or pharmacokinetics. They may alter the drug's absorption, distribution, metabolism, or excretion, potentially influencing its therapeutic effectiveness.
Are there specific stability studies conducted to evaluate impurity formation in Ivabradine?
Yes, stability studies are conducted to assess the formation of impurities in Ivabradine over time. These studies involve subjecting the drug to various storage conditions and monitoring impurity levels to ensure product stability.
Can Ivabradine impurities be formed during storage or transportation?
Impurities in Ivabradine can potentially form during storage or transportation if the drug is exposed to unfavorable conditions such as extreme temperatures, humidity, or light. Proper storage and handling procedures are essential to minimize impurity formation.
How should Ivabradine impurities be stored in terms of temperature?
The recommendation is to store Ivabradine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.