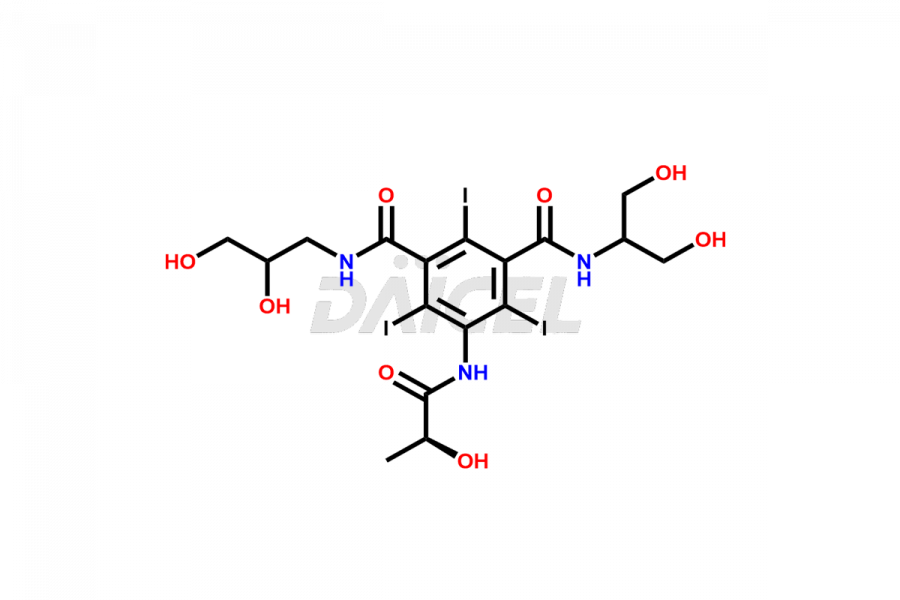
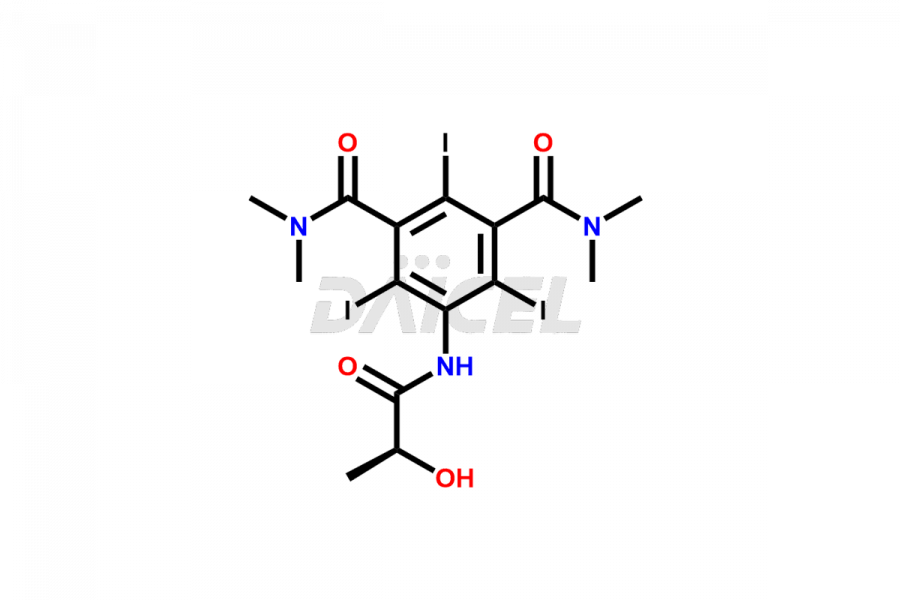
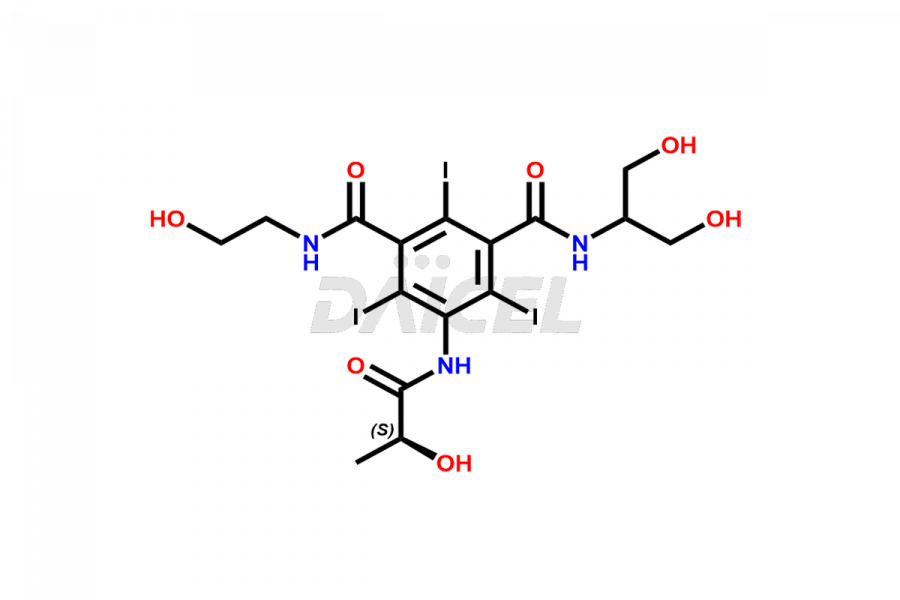
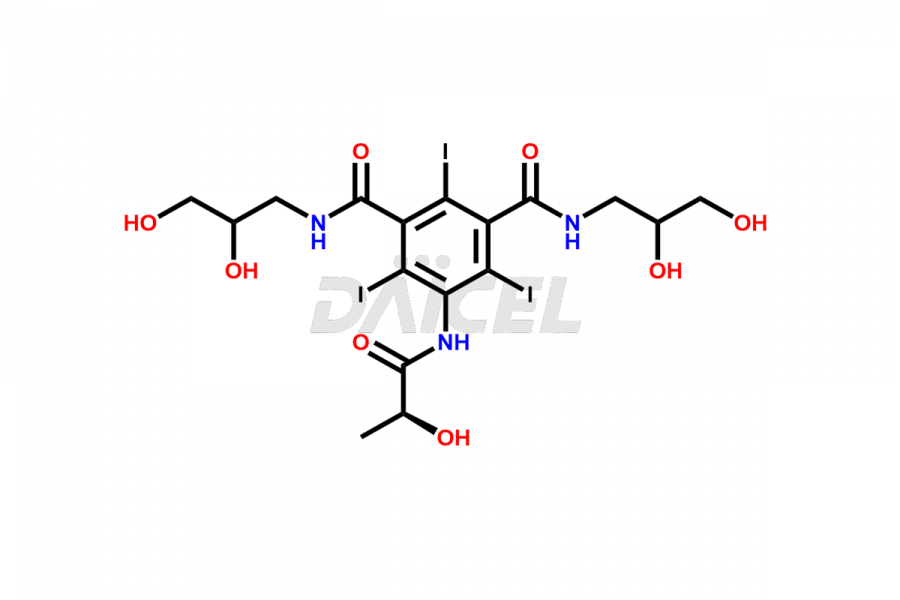
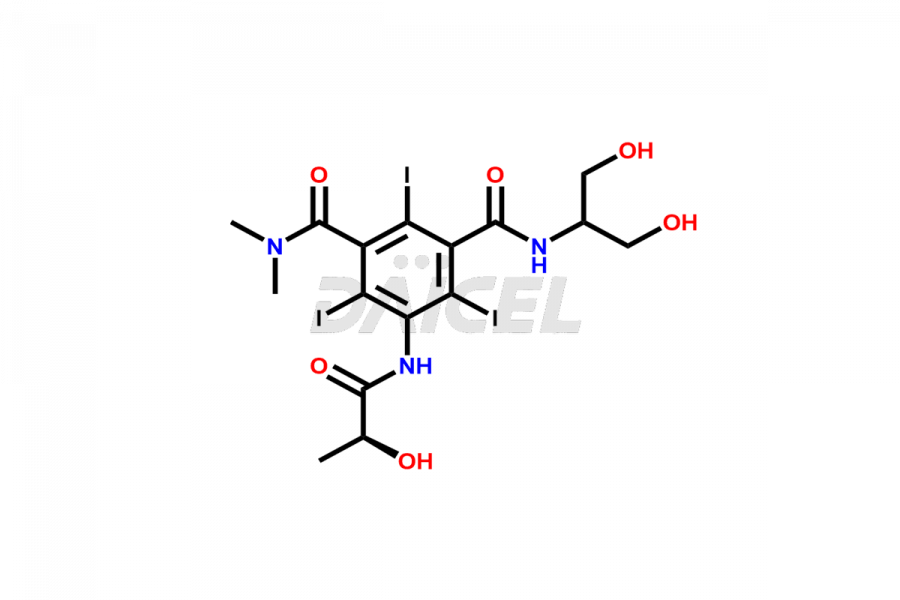
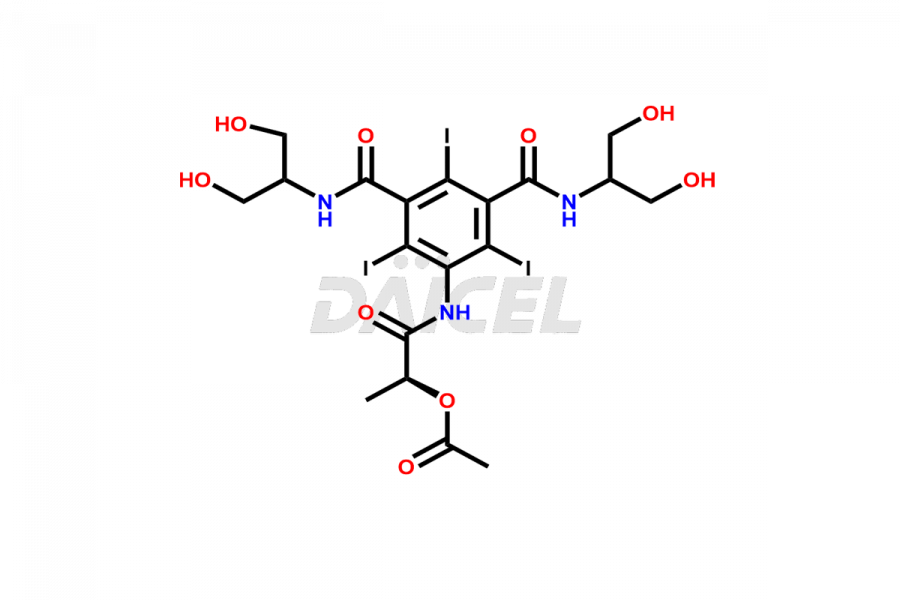
Iopamidol
General Information
Iopamidol Impurities and Iopamidol
Daicel Pharma synthesizes impurity standards for Iopamidol, an active pharmaceutical ingredient. We offer crucial impurity standards such as 2,3- Dihydroxy propyl isomer /Iso Iopamidol, Bis-Dimethylamino Iopamidol, Hydroxy ethyl derivative(or) butryl (or) EP Impurity-J, Iso Iso Iopamidol, N, N Dimethyl amino derivative, Dimethyl amino Iopamidol, Iopamidol EP Impurity-F, and O-Acetyl Iopamidol/ AP ADI, which play a vital role in evaluating the purity, and safety of Iopamidol. Daicel Pharma also gives a custom synthesis of Iopamidol impurity standards to meet specific client needs and worldwide delivery options.
Iopamidol [CAS: 60166-93-0] is a non-ionic, water-soluble contrast agent used in radiological procedures such as myelography, arthrography, nephroangiography, arteriography, etc. It’s a benzenedicarboxamide compound and acts as a radioopaque medium in these procedures.
Iopamidol: Use and Commercial Availability
Isovue-128, Isovue-200, etc., are the brands under which Iopamidol is available. It is a non-ionic, water-soluble radiographic contrast medium containing organic iodine. Iopamidol serves the purpose of blocking X-rays, allowing the visualization of body structures that lack iodine. The degree of opacity observed is directly related to the amount of the iodinated contrast agent present in the path of the X-rays. The distribution and elimination of Iopamidol play a crucial role in visualizing body structures.
Iopamidol Structure and Mechanism of Action 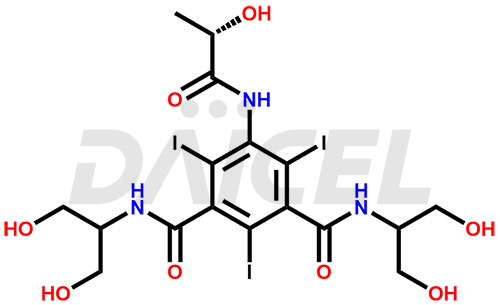
The chemical name of Iopamidol is N1, N3-Bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxy-1-oxopropyl]amino]-2,4,6-triiodo-1,3-benzenedicarboxamide. Its chemical formula is C17H22I3N3O8, and its molecular weight is approximately 777.1 g/mol.
Iopamidol enhances computed tomographic brain imaging with radiographic efficiency.
Iopamidol Impurities and Synthesis
The control and analysis of impurities in Iopamidol are crucial for ensuring its quality and safety as a radiographic contrast medium. They can arise from various sources, including the synthesis process and storage conditions. It is essential to monitor and control impurity levels at each preparation stage. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help in impurity profiling and quantification. Stringent quality control measures can meet regulatory requirements and maintain the purity and safety of Iopamidol. Regular testing and monitoring help minimize impurities and ensure the desired quality of the contrast agent.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Iopamidol impurity standards like 2,3- Dihydroxy propyl isomer /Iso Iopamidol, Bis-Dimethylamino Iopamidol, Hydroxy ethyl derivative(or) butryl (or) EP Impurity-J, Iso Iso Iopamidol, N, N Dimethyl amino derivative, Dimethyl amino Iopamidol, Iopamidol EP Impurity-F, and O-Acetyl Iopamidol/ AP ADI. We offer a comprehensive Certificate of Analysis (CoA) for these impurity standards, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Iopamidol impurity standards or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Felder, Ernst; Pitre, Davide, Water-soluble, non-ionizing hydroxy-containing amide derivatives of 2,4,6-triiodo-isophthalic acid, Savac A.-G., Switzerland, US4001323A, January 4, 1977
- Shihabi, Z. K.; Rocco, M. V.; Hinsdale, M. E., Analysis of the contrast agent iopamidol in serum by capillary electrophoresis, Journal of Liquid Chromatography, Volume: 18, Issue: 18 & 19, Pages: 3825-31, 1995
Frequently Asked Questions
Can Iopamidol impurities impact its stability?
Some impurities in Iopamidol can affect its stability. They may contribute to degradation processes, causing reduced shelf life or changes in the drug's properties. And controlling impurities helps maintain stability.
How are Iopamidol impurities identified?
Impurities in Iopamidol are identified using analytical techniques such as mass spectrometry, nuclear magnetic resonance spectroscopy, and infrared spectroscopy. These methods help in determining the chemical structure and composition of impurities.
Which solvent helps in analyzing Iopamidol impurities?
Water is commonly used as a solvent when analyzing many impurities in Iopamidol.
How should Iopamidol impurities be stored in terms of temperature?
The recommendation is to store Iopamidol impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.