LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma specializes in synthesizing top-notch impurity standards for Indacaterol, an active pharmaceutical ingredient. We offer impurity standards such as 2,3-dihydro-1H-inden-2-amine hydrochloride, Benzyl Indacaterol Impurity, DEAH_5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride, ICM-I_[(R)-8-(benzyloxy)-5-(2-bromo-1-hydroxyethyl) quinolin-2(1H)-one] Indacaterol Acetate Impurity A, Indacaterol Acetate S-Isomer, Indacaterol Impurity 8, Indacaterol monoethyl impurity, N-(5-ethyl-2,3-dihydro-1H-inden-2-yl)-2,2,2-trifluoroacetamide, and REDUCED IMP OF 5,6 DIETHYLINDENAMINE HCl, which play a vital role in evaluating the purity, and safety of Indacaterol. Daicel Pharma also provides custom synthesis of Indacaterol impurities to meet specific client needs, and we offer worldwide delivery options.
Indacaterol [CAS: 312753-06-3] is a bronchodilator agent and beta2-adrenergic agonist that treats chronic obstructive pulmonary disease (COPD). It is a monohydroxyquinoline compound and helps in managing asthma.
Arcapta Neohaler is a brand that contains Indacaterol. Indacaterol is a US FDA-approved medicine for treating chronic obstructive pulmonary disease (COPD). It combines with other bronchodilators and is administered as a dry powder. Indacaterol helps manage airflow obstruction in COPD patients, including those with chronic bronchitis and emphysema.
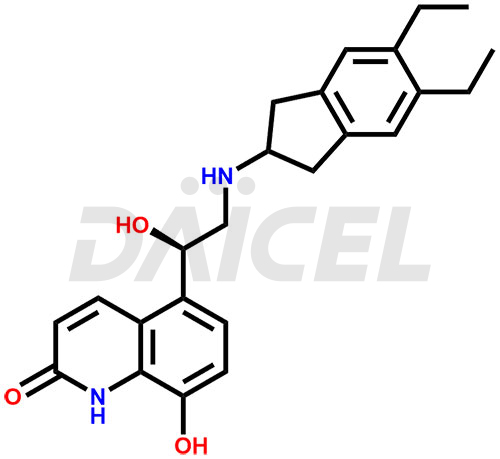
The chemical name of Indacaterol is 5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-2(1H)-quinolinone. Its chemical formula is C24H28N2O3, and its molecular weight is approximately 392.5 g/mol.
Indacaterol stimulates intracellular adenyl cyclase, the enzyme that catalyzes the conversion of ATP to cyclic monophosphate. It leads to the relaxation of bronchial smooth muscles.
The synthesis1, analysis, and control of impurities in Indacaterol are necessary for ensuring the medicine’s quality and safety. Impurities can arise during preparation or storage and can impact the efficacy and stability of the drug. Various synthetic routes produce Indacaterol and monitor and control impurities at each step. Analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), and mass spectrometry (MS) are for impurity profiling and quantification. Stringent quality control measures can meet regulatory standards and ensure the purity and safety of Indacaterol.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Indacaterol impurity standards like 2,3-dihydro-1H-inden-2-amine hydrochloride, Benzyl Indacaterol Impurity, DEAH_5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride, ICM-I_[(R)-8-(benzyloxy)-5-(2-bromo-1-hydroxyethyl) quinolin-2(1H)-one] Indacaterol Acetate Impurity A, Indacaterol Acetate S-Isomer, Indacaterol Impurity 8, Indacaterol monoethyl impurity, N-(5-ethyl-2,3-dihydro-1H-inden-2-yl)-2,2,2-trifluoroacetamide, and REDUCED IMP OF 5,6 DIETHYLINDENAMINE HCl. We offer a comprehensive Certificate of Analysis (CoA) for these impurity standards, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Indacaterol impurity standards and degradation products. Each delivery has a comprehensive characterization report.
Optimization techniques, including process modifications and purification steps, help minimize the formation of impurities during the preparation of Indacaterol.
Proper storage conditions, including temperature and humidity control, help prevent degradation and impurity formation in Indacaterol.
Impurities in Indacaterol require rigorous quality control practices, ongoing stability testing, and adherence to regulatory guidelines from early development to post-marketing surveillance.
Indacaterol impurities storage should be at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.