Imatinib
General Information
Imatinib Impurities and Imatinib
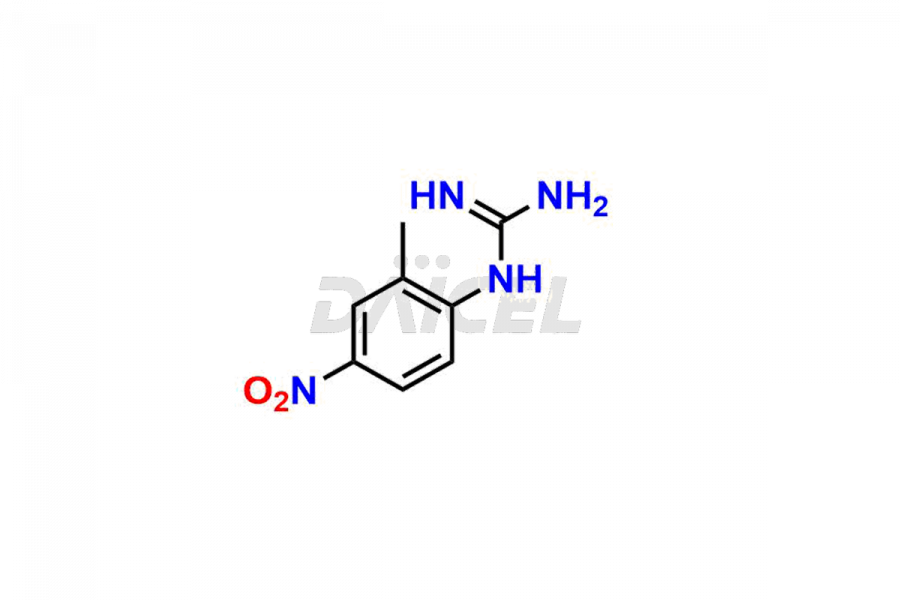
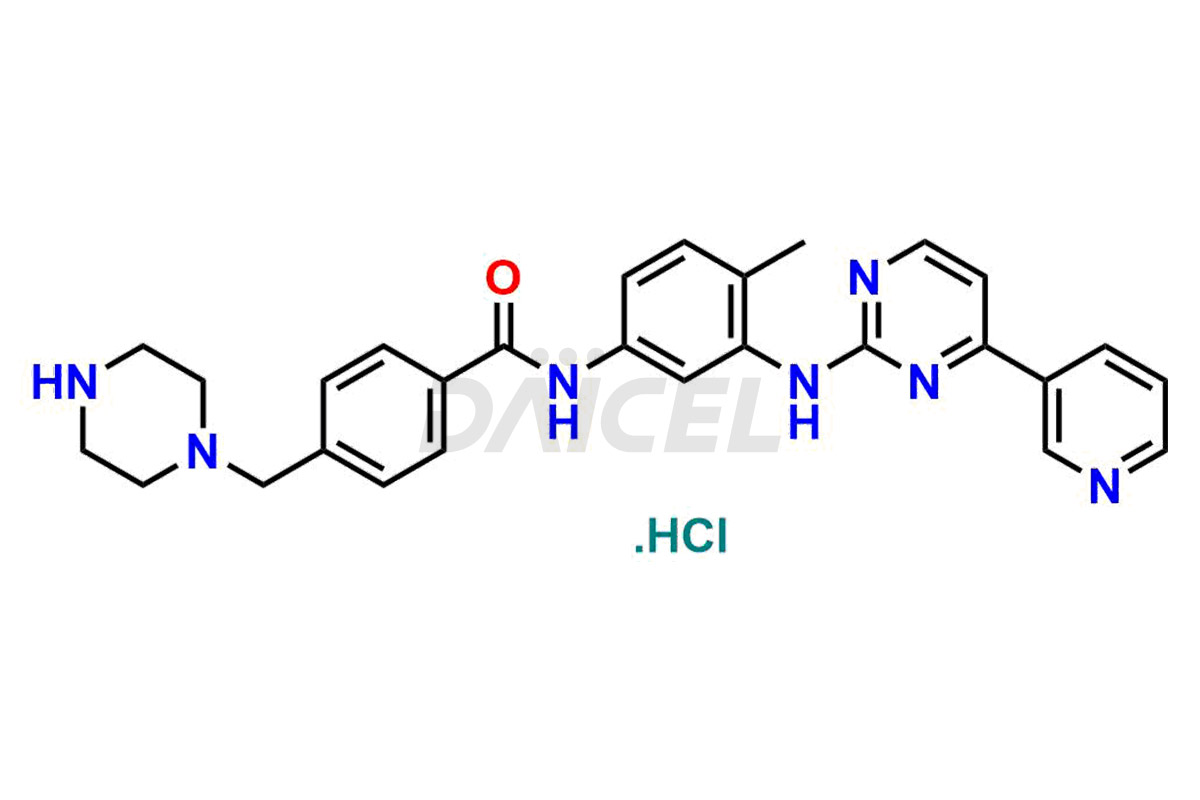
Daicel Pharma offers worldwide delivery options for a custom synthesis of Imatinib impurities, including impurities such as 1-(2-methyl-3-nitrophenyl)guanidine, 1-(2-methyl-4-nitrophenyl)guanidine, 1-(2-methyl-5-nitrophenyl)guanidine Nitrate, N-(2-Methyl-5-nitrophenyl)-N’-Carbamoyl Guanidine, and N-Desmethyl Imatinib. These impurities play a vital role in evaluating the purity and safety of Imatinib, an active pharmaceutical ingredient.
Used as its mesylate salt, Imatinib [CAS: 152459-95-5] is a benzamide and a small molecule kinase inhibitor for treating cancer. It treats chronic myelogenous leukemia and gastrointestinal stromal tumors caused by abnormal, constitutively expressed tyrosine kinases that drive unregulated cell growth.
Imatinib: Use and Commercial Availability
Imatinib mesylate (Imatinib), or Gleevec, is an oral tyrosine kinase inhibitor approved by the US FDA for treating chronic myelogenous leukemia. It has also received approval for several other oncologic conditions. They include various stages of Philadelphia chromosome-positive chronic myelogenous leukemia, relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia, myelodysplastic/myeloproliferative diseases with specific gene rearrangements, aggressive systemic mastocytosis, hypereosinophilic syndrome/chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, unresectable/metastatic gastrointestinal stromal tumors (GIST), and adjuvant treatment following resection of Kit-positive GIST.
Imatinib Structure and Mechanism of Action 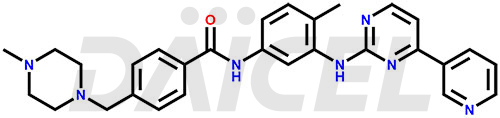
The chemical name of Imatinib is 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide. Its chemical formula is C29H31N7O, and its molecular weight is approximately 493.6 g/mol.
Imatinib induces apoptosis and inhibits Bcr-Abl tyrosine kinase activity. Philadelphia chromosome abnormality in chronic myeloid leukemia (CML) creates abnormal tyrosine kinase.
Imatinib Impurities and Synthesis
Imatinib can have impurities that may be present due to various factors during its synthesis1 and storage. Common Imatinib impurities include related substances from incomplete synthesis or degradation processes, organic impurities from starting materials or intermediates, inorganic impurities such as heavy metals or salts, and residual solvents used during manufacturing. Regulatory authorities have established limits for impurity levels to ensure the quality and safety of Imatinib. Stringent quality control measures implemented by manufacturers minimize impurities and meet regulatory requirements for the final product.
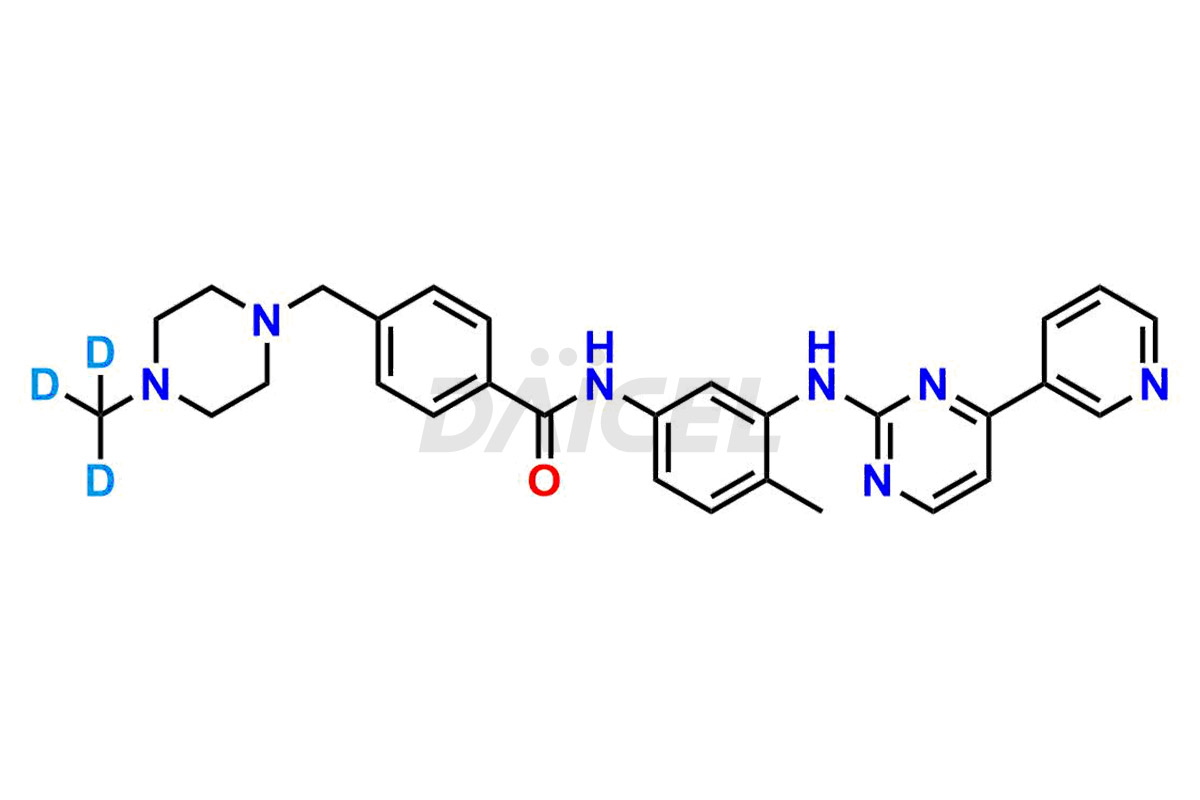
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Imatinib impurity standards such as 1-(2-methyl-3-nitrophenyl)guanidine, 1-(2-methyl-4-nitrophenyl)guanidine, 1-(2-methyl-5-nitrophenyl)guanidine Nitrate, N-(2-Methyl-5-nitrophenyl)-N’-Carbamoyl Guanidine, and N-Desmethyl Imatinib. We offer deuterium-labeled Imatinib standard, Imatinib-D3, essential for bioanalytical research and BA/BE studies. Our impurities have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Imatinib impurities, degradation products, and labeled compounds. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Zimmermann, Juerg, Pyrimidin derivatives and process for their preparation, Ciba-Geigy A.-G., Switzerland, EP564409B1, January 19, 2001
- Widmer, N.; Beguin, A.; Rochat, B.; Buclin, T.; Kovacsovics, T.; Duchosal, M. A.; Leyvraz, S.; Rosselet, A.; Biollaz, J.; Decosterd, L. A., Determination of imatinib (Gleevec) in human plasma by solid-phase extraction-liquid chromatography-ultraviolet absorbance detection, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 803, Issue: 2, Pages: 285-292, 2004
Frequently Asked Questions
How do regulatory authorities monitor and ensure compliance with impurity limits in Imatinib?
Regulatory authorities conduct inspections of manufacturing facilities, review data from quality-control tests and assess compliance with regulatory guidelines to monitor impurity levels in Imatinib. They may also conduct post-market surveillance and sampling to verify ongoing compliance.
Are there any specific Imatinib impurities that require special attention?
Some impurities in Imatinib, such as genotoxic impurities, receive special attention due to their potential to cause DNA damage and adverse health effects. Manufacturers have robust control strategies to minimize genotoxic impurities and ensure their levels are within acceptable limits.
Which solvent help in analyzing Imatinib impurities?
DMSO or water are the solvents commonly used when analyzing many impurities in Imatinib.
How should Imatinib impurities be stored in terms of temperature?
The recommendation is to store Imatinib impurities at room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.