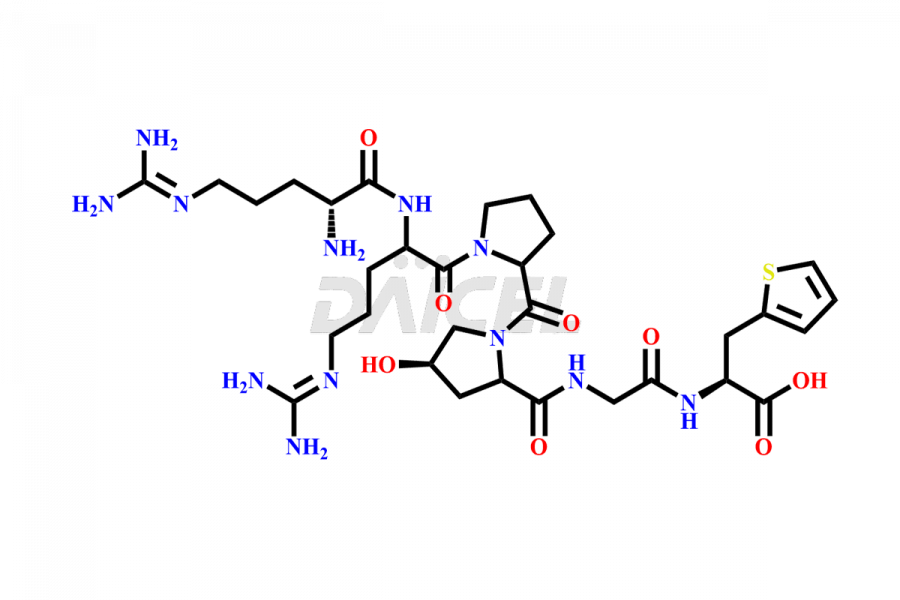
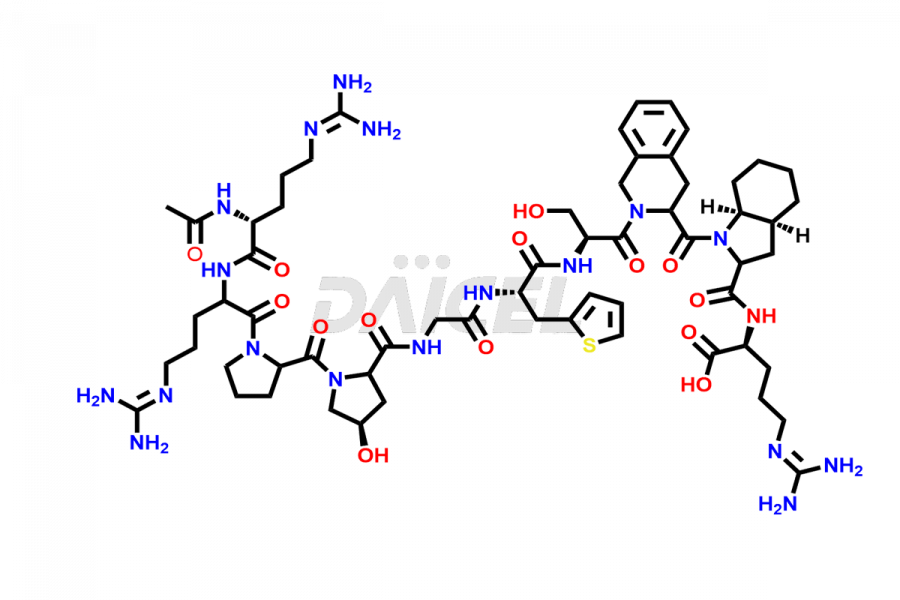
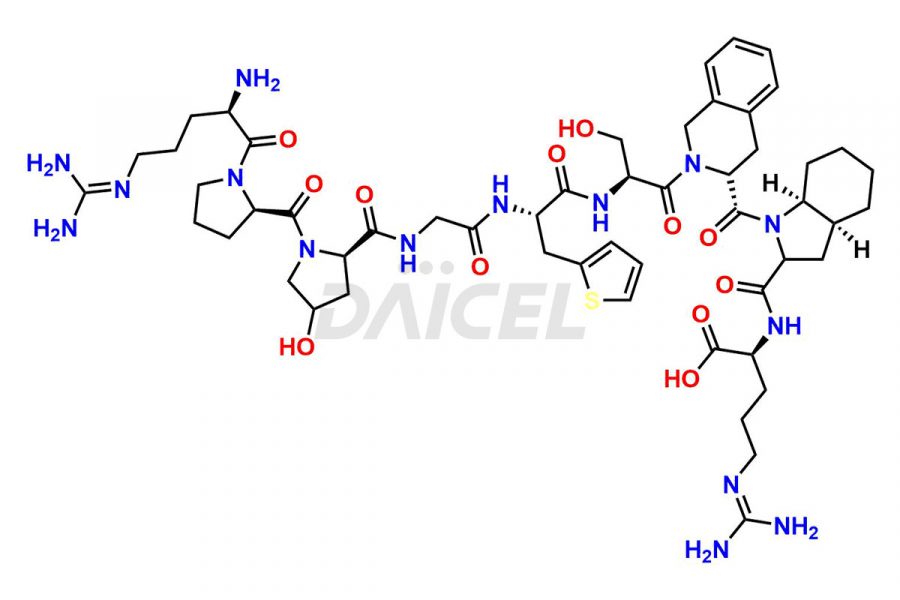
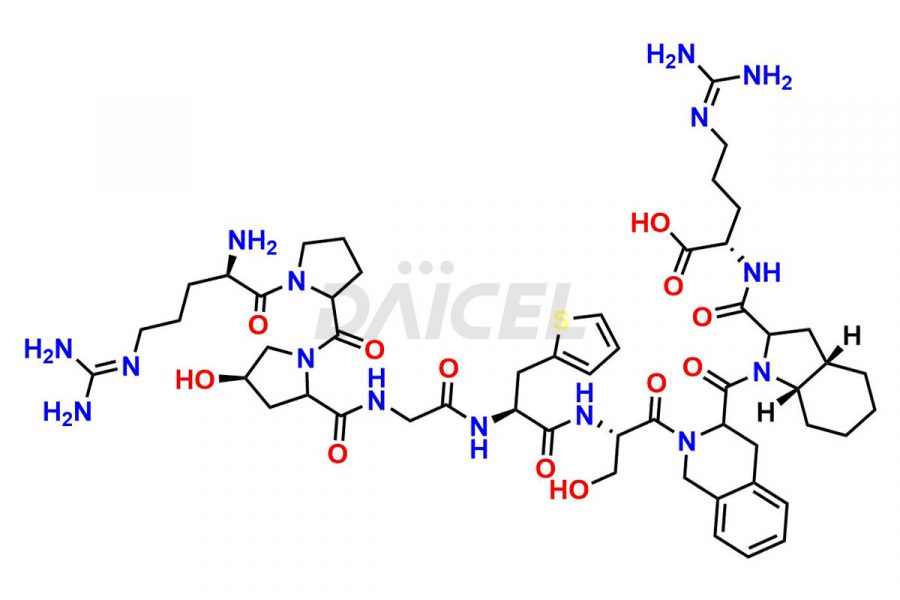
Icatibant
General Information
Icatibant Impurities and Icatibant
Daicel Pharma synthesizes high-quality Icatibant impurities, (1-6)-Icatibant, (7-10)-Icatibant, Ac-Icatibant, Des-D-Arg(1)-Icatibant, Des-L-Arg(2)-Icatibant, and L-Arg(1)-Icatibant, that help in the quality, stability, and biological safety analysis of Icatibant. We also offer custom synthesis of Icatibant impurities and supply worldwide.
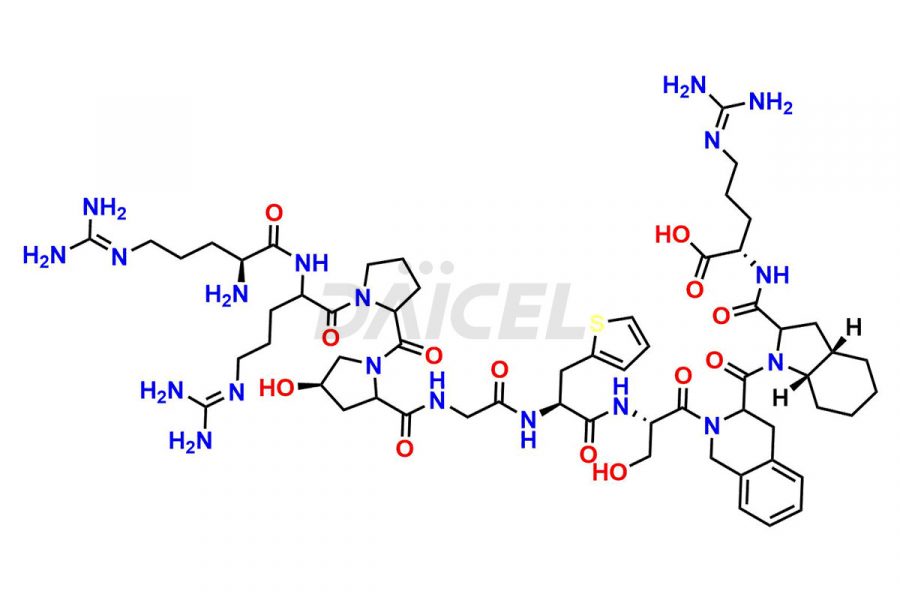
Icatibant [CAS: 130308-48-4] is a synthetic peptidomimetic consisting of ten amino acids, which is a selective and specific antagonist of bradykinin B2 receptors. It is used for the symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults with C1-esterase-inhibitor deficiency.
Icatibant: Use and Commercial Availability
Icatibant, a drug, is a competitive antagonist selective for bradykinin B2 receptor (B2R), and commonly prescribed to treat acute attacks of swelling and inflammation in individuals with hereditary angioedema (HAE). It is under research for its potential to treat angioedema, joint disease, and chronic kidney disease. Icatibant is marketed under the brand names Firazyr and Sajazir.
Icatibant Structure and Mechanism of Action
The chemical formula for Icatibant is C59H89N19O13 and its molecular weight is approximately 1304.5 g/mol.
A C1-esterase inhibitor is the main regulator of the Factor XII I/kallikrein proteolytic cascade that leads to bradykinin production. The absence or dysfunction of a C1-esterase inhibitor causes Hereditary angioedema (HAE). Bradykinin is responsible for the characteristic HAE symptoms of localized swelling, inflammation, and pain. Icatibant prevents bradykinin from binding the B2 receptors and treats acute attacks of HAE.
Icatibant Impurities and Synthesis
Icatibant is synthesized1 from the protected L- and D- amino acids and protected glycine. The synthesis is by a solid phase peptide synthesis (SPPS) process. Icatibant may contain impurities2 such as related peptide impurities, organic impurities, residual solvents, and degradation products. These impurities must be monitored and controlled during the manufacturing process to ensure that the final product meets the quality standards.
At Daicel, we provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Icatibant impurity standards, (1-6)-Icatibant, Icatibant (7-10), Ac-Icatibant, Des-D-Arg(1)-Icatibant, Des-L-Arg(2)-Icatibant, and L-Arg(1)-Icatibant, along with complete characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Upon request, we provide 13C-DEPT and CHN. Also, we provide a complete characterization report upon delivery. Daicel offers highly pure isotope-labeled standards of Icatibant in bioanalytical research and BA/BE studies with isotope data in CoA.
References
FAQ's
References
- Stephan Henke; Hiristo Anagnostopulos; Gerhard Breipohl; Jochen Knolle; Jens Stechl,; Bemward Scholkens; Hans-Wolfram Fehlhaber; Hermann Gerhards; Franz Hock; Hoechst Aktiengesellschaf, “Peptides Having Bradykevin Antagonist Action” US patent US5648333, July 15, 1997
- Gupta, Praveer; Siriki, Yernaidu; Sadhanala, Trimurthulu; Akula, Ravi Kumar; Alembic Pharmaceuticals Limited, “An improved process for preparation of icatibant by solid phase peptide synthesis and its acetate salt” Indian publication, IN201621021157, June 21, 2016
- Lajin, Bassam; Steiner, Oliver; Fasshold, Lisa; Zangger, Klaus; Goessler, Walter, “The identification and chromatographic separation of a new highly analogous impurity of the active pharmaceutical ingredient icatibant”, European Journal of Pharmaceutical Sciences, Volume: 132, Pages: 121-124, 2019
Frequently Asked Questions
What are the acceptable limits for related peptide Icatibant impurities?
The acceptable limit for related peptide Icatibant impurities is typically 0.5% or less.
How are related peptide Icatibant impurities synthesized?
Related peptide Icatibant impurities are synthesized using the same methods and starting materials as Icatibant itself. Further, they are separated from the desired product during the purification process.
What are the common methods used to synthesize organic Icatibant impurities?
Organic Icatibant impurities form through various chemical reactions, including oxidation, reduction, and cyclization. The methods used to synthesize these impurities will depend on the specific, targeted impurity.
What analytical methods are used to monitor impurities during the synthesis of Icatibant?
Analytical methods such as high-performance liquid chromatography3 (HPLC), and liquid chromatography mass spectrometry (LC-MS) are commonly used to monitor impurities during the synthesis of Icatibant. These methods allow for the identification and quantification of impurities at various stages of the synthetic process.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.