LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma synthesizes more than ten high-quality Hydrocortisone impurities such as Keto Hydroxyprogesteron-17, Cortisone 17-Carboxylic Acid, Hydrocortisone (9B, 11B – Epoxide), Hydrocortisone 17-Formoxyl Impurity, Hydrocortisone E-enol aldehyde, Hydrocortisone Z-enol aldehyde, Hydrocortisone-20-acid and more, crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Hydrocortisone. Moreover, Daicel Pharma offers custom synthesis of Hydrocortisone impurities and delivers them globally.
Hydrocortisone [CAS: 50-23-7] is a medicine similar in chemical structure to the natural hormone produced by the adrenal glands. It is a synthetic or semisynthetic analog of the natural hydrocortisone hormone. It helps treat various medical conditions, including immune system dysfunction, cancer, inflammation, etc.
Hydrocortisone is a versatile medication treating various medical conditions affecting collagen, endocrine, allergic, rheumatic, hematologic, respiratory, gastrointestinal, neoplastic, ophthalmic, etc. Based on the patient’s condition Hydrocortisone is available in various forms, including tablets, enemas, topical ointments with antibiotics, and topical creams with acyclovir for cold sores. Hydrocortisone is marketed under different brand names worldwide, including Cortef, Solu-Cortef, and Hydrocortone.
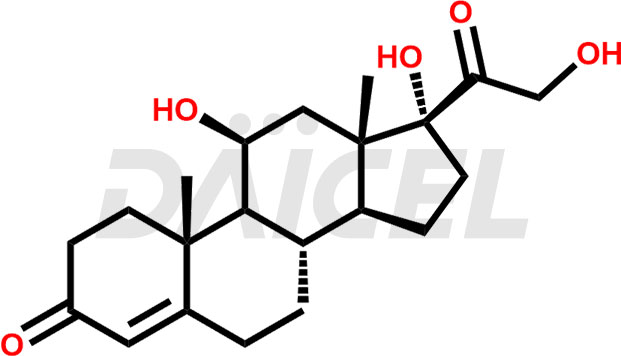
The chemical name of Hydrocortisone is 11β,17,21-Trihydroxypregn-4-ene-3,20-dione. Its chemical formula is C21H30O5, and its molecular weight is approximately 362.5g/mol.
Hydrocortisone inhibits the accumulation of inflammatory cells at inflammation sites. It binds to glucocorticoid receptors and inhibits inflammatory transcription factors.
When Hydrocortisone is manufactured1, impurities2 may form substances that may potentially harm patients. Some impurities that can develop include 11- epimers and degradation products generated from hydrolysis or oxidation. It is necessary to monitor impurity levels in Hydrocortisone to maintain its safety and effectiveness in treating patients.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Hydrocortisone impurity standards, including Keto Hydroxyprogesteron-17, Cortisone 17-Carboxylic Acid, Hydrocortisone (9B, 11B – Epoxide), Hydrocortisone 17-Formoxyl Impurity, Hydrocortisone E-enol aldehyde, Hydrocortisone Z-enol aldehyde, Hydrocortisone-20-acid and so on. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Hydrocortisone impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Hydrocortisone. Daicel offers highly pure isotope-labeled standards of Hydrocortisone for bioanalytical research and BA/BE studies with isotope data in CoA.
Analytical methods such as high-performance liquid chromatography (HPLC) help identify and quantify Hydrocortisone impurities.
Purification methods involving chromatography remove impurities from Hydrocortisone .
Some sources of Hydrocortisone impurities include starting materials, intermediates, and degradation products.
Hydrocortisone impurities may cause harm to humans if they have high toxicity levels.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.