Granisetron
General Information
Granisetron Impurities and Granisetron
Daicel Pharma offers worldwide delivery options for a custom synthesis of Granisetron impurities, including the impurity standard, 7-Methoxy Granisetron. The impurity plays a vital role in evaluating the purity and safety of Granisetron, an active pharmaceutical ingredient.
An indazole derivative, Granisetron [CAS: 109889-09-0], serves as a selective antagonist of the 5-HT3 receptor, effectively managing nausea and vomiting caused by cancer chemotherapy and radiotherapy. Acting as an antiemetic, Granisetron functions as a serotonin receptor antagonist. Its effectiveness in alleviating these symptoms has made it a widely prescribed medication in clinical practice.
Granisetron: Use and Commercial Availability
Granisetron, available under brand names like Granisol, Kytril, Sancuso, and Sustol, is a medication to prevent nausea and vomiting. It is for individuals undergoing emetogenic cancer therapy, including high-dose cisplatin, post-operation, and radiation treatments. Additionally, Granisetron prevents nausea and vomiting during moderately or highly emetogenic chemotherapy, whether with or without cisplatin, for up to five consecutive days.
Granisetron Structure and Mechanism of Action 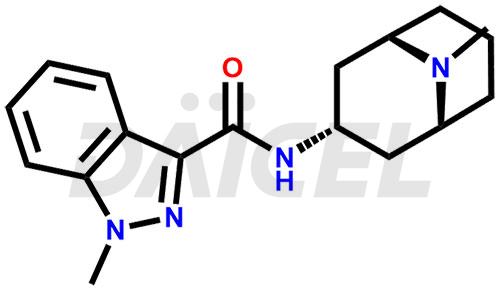
The chemical name of Granisetron is 1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide. Its chemical formula is C18H24N4O, and its molecular weight is approximately 312.4 g/mol.
Granisetron is a 5-hydroxytryptamine3 (5-HT3) receptor antagonist which binds with serotonin receptors located on the vagal nerve terminals peripherally and centrally in the chemoreceptor zone.
Granisetron Impurities and Synthesis
Granisetron impurities can form during the synthesis1, storage, or usage of the drug due to side reactions, incomplete reactions, or interactions with other compounds. Analytical methods such as HPLC, LC-MS, NMR, and IR spectroscopy help analyze and quantify these impurities. The control measures involve optimizing the synthetic process and purification steps and ensuring appropriate storage conditions to minimize impurity formation. Stability studies assess impurity profiles over time and establish expiration dates and storage recommendations. Compliance with regulatory guidelines, such as those set by ICH, is crucial for ensuring the quality and safety of Granisetron formulations. Effective impurity control measures contribute to maintaining the integrity of Granisetron as a pharmaceutical product.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility synthesizing Granisetron impurity standard, 7-Methoxy Granisetron. We offer deuterium-labeled Granisetron standards, including 7-Hydroxy Granisetron-D3 and Granisetron-D3, essential for bioanalytical research and BA/BE studies. Our impurities include a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report has data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Granisetron impurities or degradation products and labeled compounds. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Bermudez, Jose; Fake, Charles S.; Joiner, Graham F.; Joiner, Karen A.; King, Frank D.; Miner, Wesley D.; Sanger, Gareth J., 5-Hydroxytryptamine (5-HT3) receptor antagonists. 1. Indazole and indolizine-3-carboxylic acid derivatives, Journal of Medicinal Chemistry, Volume: 33, Issue: 7, Pages: 1924-9, 1990
- Kudoh, Shinobu; Sato, Tomoyuki; Okada, Hideki; Kumakura, Hiroyuki; Nakamura, Hiroshi, Simultaneous determination of granisetron and 7-hydroxygranisetron in human plasma by high-performance liquid chromatography with fluorescence detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 660, Issue: 1, Pages: 205-10, 1994
Frequently Asked Questions
Are Granisetron impurities regulated differently in different countries?
While there may be variations in impurity regulations between countries, the fundamental objective remains the same: to ensure the safety and quality of Granisetron.
How are Granisetron impurities classified?
Impurities in Granisetron are classified as organic or inorganic impurities, related substances, or degradation products based on their origin and chemical nature.
Can Granisetron impurities interact with other medications?
Some impurities in Granisetron may have the potential to interact with other medications, which highlights the importance of considering potential drug interactions during treatment.
How should Granisetron impurities be stored in terms of temperature?
The recommendation is to store Granisetron impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.




