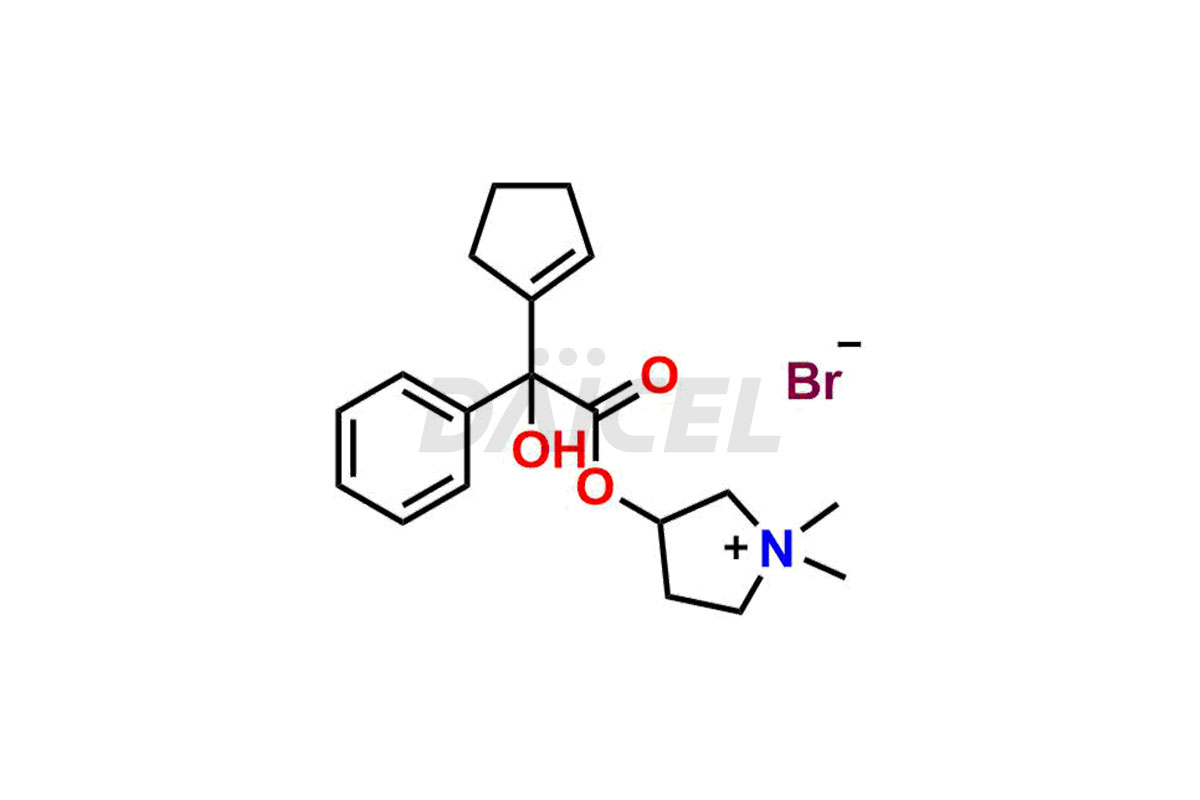
Glycopyrronium
General Information
Glycopyrronium Impurities and Glycopyrronium
Daicel Pharma offers worldwide delivery options for a custom synthesis of Glycopyrronium impurities, including crucial impurities such as Chloro Glycopyrronium Bromide (Imp-I), Dehydro Glycopyrronium Bromide, Glycopyrrolate Related Compound C, and Glycopyrronium Impurity B. These impurities play a vital role in evaluating the purity and safety of Glycopyrronium, an active pharmaceutical ingredient.
Glycopyrronium [CAS: 596-51-0], a synthetic quaternary ammonium compound, is an anticholinergic agent with antispasmodic and antisecretory properties. It treats gastrointestinal conditions characterized by intestinal spasms and the reduction of excessive secretions during anesthesia. As a muscarinic antagonist, Glycopyrronium effectively alleviates spasms in gastrointestinal disorders and aids in controlling salivation when used alongside specific anesthetics.
Glycopyrronium: Use and Commercial Availability
Glycopyrronium, or glycopyrrolate, is an anticholinergic medication with a quaternary amine structure. It is commonly used before surgery to inhibit the salivary glands secretion and respiratory system. Anticholinergics like Glycopyrronium achieve effects such as reducing saliva production, inducing sedation and amnesia, and preventing reflex bradycardia. However, their impact on gastric fluid pH and volume is not consistent. Qbrexza is the brand name under which Glycopyrronium is available.
The chemical name of Glycopyrronium is 3-[(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethyl- Pyrrolidinium bromide. Its chemical formula is C19H28BrNO3, and its molecular weight is approximately 398.3 g/mol.
Glycopyrronium blocks acetylcholine effects at the parasympathetic sites in the central nervous system, secretory glands, and smooth muscle tissues.
Glycopyrronium Impurities and Synthesis
Impurities in Glycopyrronium can arise during synthesis1, storage, or degradation processes. Analytical techniques such as HPLC, LC-MS, NMR, and IR spectroscopy help in impurity analysis. Control measures include optimizing synthesis conditions, implementing purification steps, and ensuring proper storage. Stability studies assess impurity profiles and guide expiration dates and storage recommendations. Compliance with regulatory guidelines, such as those from ICH, is crucial. Effective impurity control measures ensure the quality and safety of Glycopyrronium.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility synthesizing Glycopyrronium impurity standards. We provide a range of Glycopyrronium impurity standards, such as Chloro Glycopyrronium Bromide (Imp-I), Dehydro Glycopyrronium Bromide, Glycopyrrolate Related Compound C, and Glycopyrronium Impurity B. Our impurities have a detailed Certificate of Analysis (CoA) that includes a comprehensive characterization report. The CoA encompasses data obtained through various techniques, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis. We give additional data, such as 13C-DEPT, on request. We can synthesize unknown Glycopyrronium impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Lunsford, Carl D., Esters of amino alcohols, A. H. Robins Co., Inc., US2956062A, October 11, 1960
- Murray, G. R.; Calvey, T. N.; Williams, N. E.; Chan, K., Quantitative capillary column gas chromatographic method for the determination of glycopyrronium in human plasma, Journal of Chromatography, Biomedical Applications, Volume: 308, Pages: 143-51, 1984
Frequently Asked Questions
Can impurities impact the effectiveness of Glycopyrronium?
Impurities can affect the effectiveness of Glycopyrronium by altering its pharmacological properties or interacting with the drug's intended mechanism of action.
Are Glycopyrronium impurities tested during clinical trials?
Yes, impurities in Glycopyrronium are monitored and assessed during the clinical trial phase to evaluate their impact on safety and efficacy.
Which solvent helps in analyzing Glycopyrronium impurities?
Methanol or Water is the common solvent used for analyzing many Glycopyrronium impurities.
How should Glycopyrronium impurities be stored in terms of temperature?
The recommendation is to store Glycopyrronium impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.