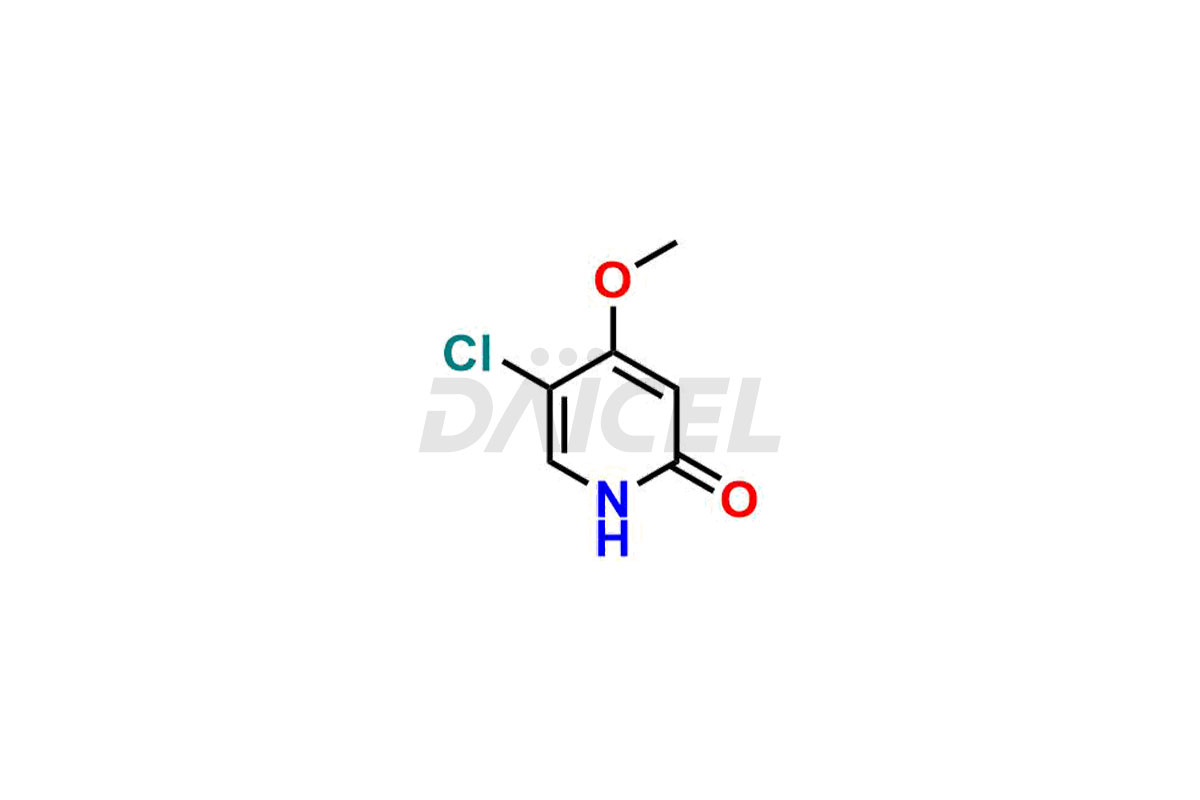
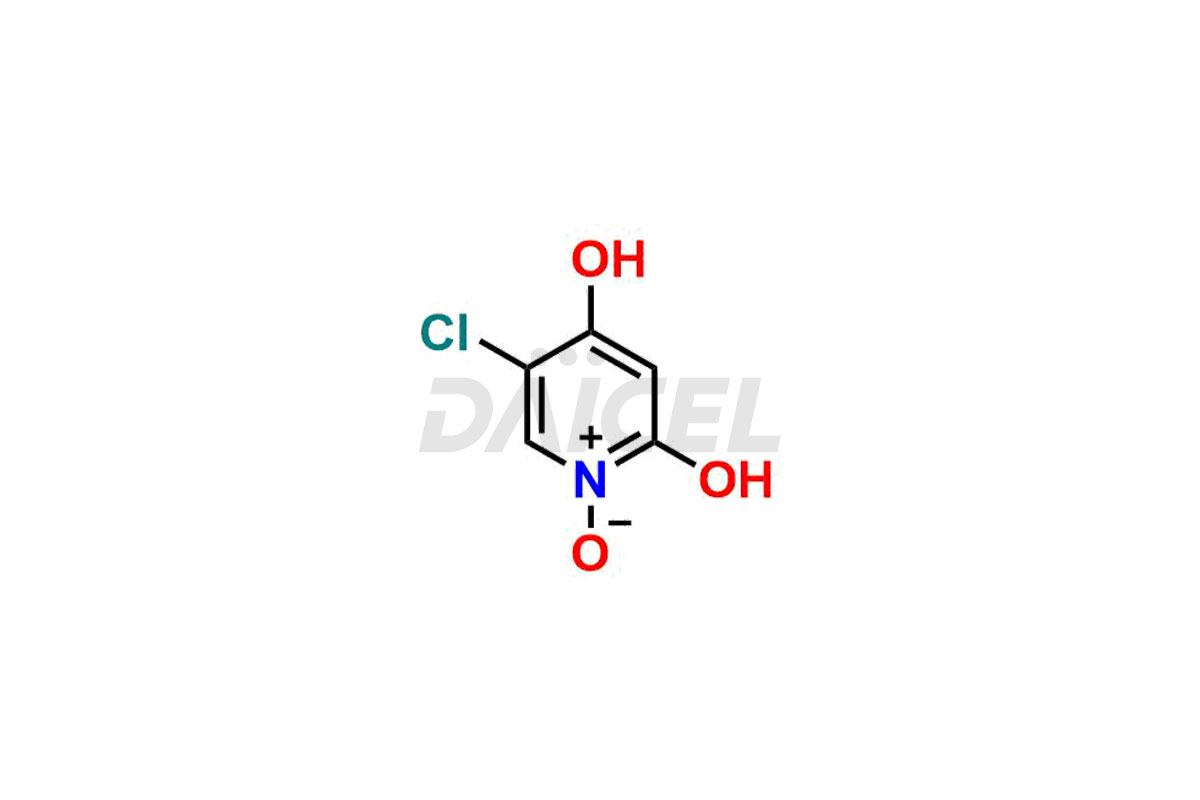
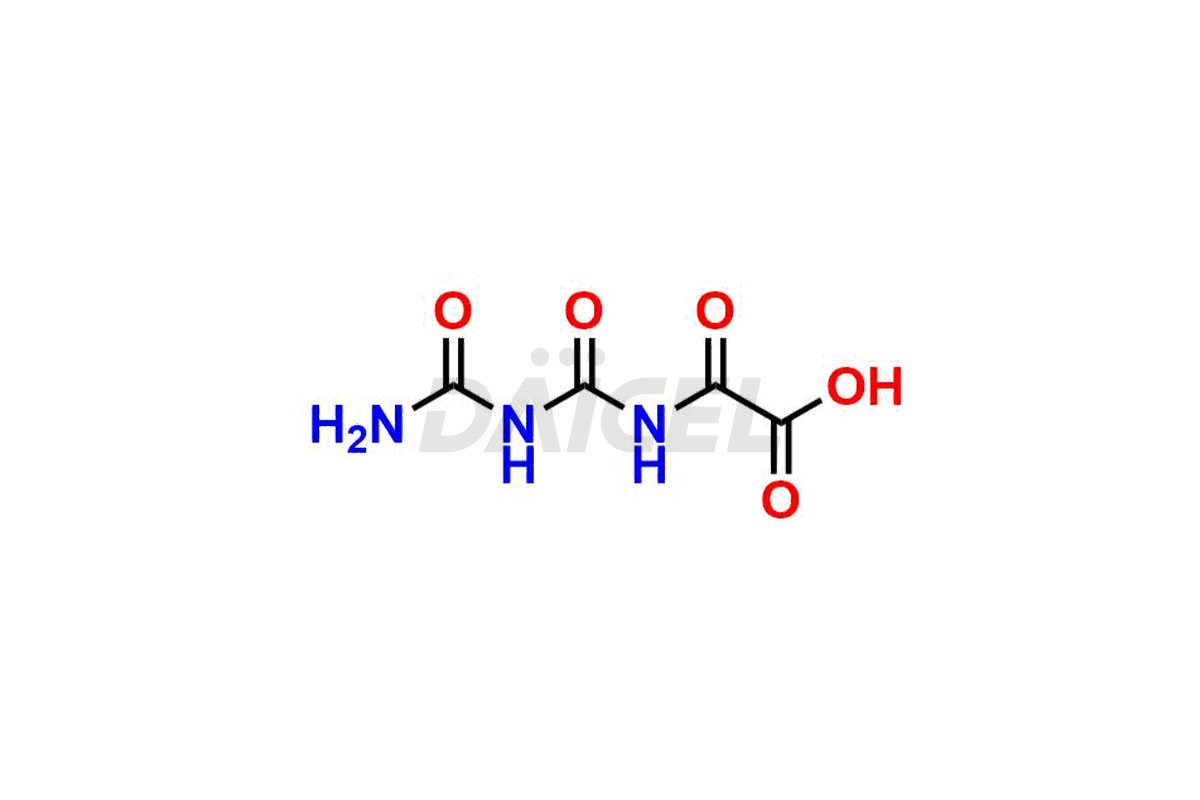
Gimeracil
General Information
Gimeracil Impurities and Gimeracil
Daicel Pharma specializes in synthesizing impurities for Gimeracil, an active pharmaceutical ingredient. We offer crucial impurities such as 5-Chloro-4-methoxy-2(1H)-pyridinone / Gimeracil Impurity 7, Gimeracil Impurity 16, and Gimeracil Impurity 3, which play a vital role in evaluating the purity, and safety of Gimeracil. Daicel Pharma also provides custom synthesis of Gimeracil impurities to meet specific client needs and offer worldwide delivery options.
By inhibiting the enzyme dihydropyrimidine dehydrogenase (DPD), Gimeracil [CAS: 103766-25-2], a pyridine derivative, effectively enhances the antitumor activity of fluoropyrimidines. Gimeracil helps to increase the effectiveness of fluoropyrimidine-based drugs by preventing their breakdown in the body.
Gimeracil: Use and Commercial Availability
When combined with cisplatin, Gimeracil, as part of the product Teysuno, treats advanced gastric (stomach) cancer in adults. It is a part of antineoplastic therapy.
Gimeracil Structure and Mechanism of Action 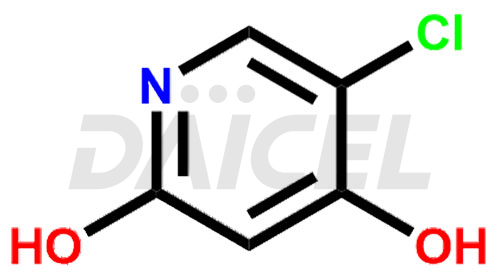
The chemical name of Gimeracil is 5-Chloro-4-hydroxy-2(1H)-pyridinone. Its chemical formula is C5H4ClNO2, and its molecular weight is approximately 145.54 g/mol.
Gimeracil prevents the breakdown of 5-FU, which acts against cancer cells. It blocks the enzyme dihydropyrimidine dehydrogenase (DPD) responsible for the degradation of 5-FU.
Gimeracil Impurities and Synthesis
During the synthesis1 of Gimeracil, various impurities may arise. Some common impurities include related pyridine derivatives, byproducts resulting from reaction side reactions, or incomplete conversion of starting materials. They can potentially impact the purity, stability, and efficacy of Gimeracil. Therefore, stringent quality control measures help monitor and control impurity levels during the synthetic process.
Daicel Pharma, in adherence to cGMP standards, has an analytical facility where we prepare Gimeracil impurities like 5-Chloro-4-methoxy-2(1H)-pyridinone / Gimeracil Impurity 7, Gimeracil Impurity 16 and Gimeracil Impurity 3. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we provide additional data like 13C-DEPT. We can synthesize unknown Gimeracil impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
Frequently Asked Questions
How does the presence of Gimeracil impurities affect the drug's shelf life?
The presence of impurities in Gimeracil can contribute to its degradation over time, potentially reducing its shelf life. Therefore, proper storage conditions, including temperature and humidity control, are essential to maintain the stability and quality of Gimeracil throughout its designated shelf life.
Can Gimeracil impurities lead to drug interactions or complications with other medications?
Drug interactions or complications caused by impurities in Gimeracil are unlikely. However, they can impact the quality and efficacy of the drug, which may indirectly affect drug interactions or therapeutic outcomes when Gimeracil is used with other medications.
How often are impurity levels in Gimeracil monitored during the manufacturing process?
Impurity levels in Gimeracil are continuously monitored throughout the manufacturing process to ensure compliance with regulatory standards. Regular testing and analysis are performed at various stages to maintain the desired purity and quality of the drug.
How should Gimeracil impurities be stored in terms of temperature?
The recommendation is to store Gimeracil impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.