LOAD MORE
You're viewed 9 of 13 products
Daicel Pharma synthesizes high-quality Gefitinib impurities like O-Desmethyl Gefitinib, Gefitinib 3,4-Difluoro Impurity, Gefitinib Impurity 13 (Deschloro impurity), Gefitinib EP Impurity B, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Gefitinib. Moreover, Daicel Pharma offers custom synthesis of Gefitinib impurities and delivers them globally.
Gefitinib [CAS: 184475-35-2] is an anilinoquinazoline compound to fight cancer. It treats non-small cell lung cancer. It is an epidermal growth factor kinase inhibitor and an antineoplastic agent.
Gefitinib treats advanced non-small cell lung cancer (NSCLC) and other types of cancer. The drug is available under the brand name Iressa.
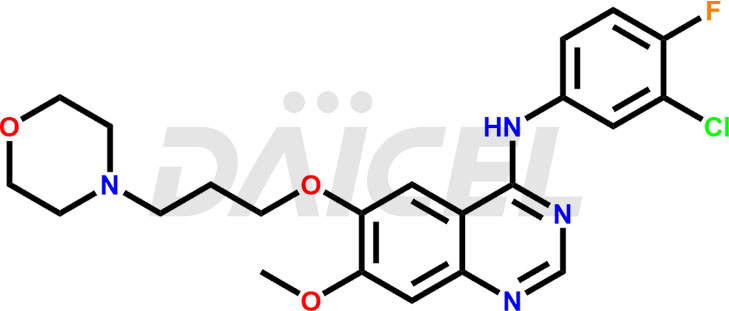
The chemical name of Gefitinib is N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine. Its chemical formula is C22H24ClFN4O3, and its molecular weight is approximately 446.9 g/mol.
Gefitinib inhibits tyrosine-kinases associated with the epidermal growth factor receptor (EGFR-TK), thus inhibiting the growth of cancer cells. However, the clinical antitumor action of Gefitinib is not fully characterized.
Gefitinib impurities can be formed during manufacturing1 or storage. The impurities include degradation products, starting materials, and other process-related impurities. Their formation can affect the quality, safety, and efficacy of the drug, and thus their identification and control are critical in drug development and manufacturing. Various analytical techniques, such as HPLC and LC-MS, helps detect and quantify impurities in Gefitinib.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Gefitinib impurity standards, O-Desmethyl Gefitinib, Gefitinib 3,4-Difluoro Impurity, Gefitinib Impurity 13 (Deschloro impurity), Gefitinib EP Impurity B, and so on. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN if requested. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Gefitinib impurity or degradation product. We also provide labeled compounds3 to quantify the efficacy of Gefitinib. Daicel offers Gefitinib-D3, a deuterium-labeled Gefitinib standard used in bio-analytical research, such as BA/BE studies with isotope data in CoA.
Impurity profiling is an essential tool in the quality control of the Gefitinib drug substance to identify, monitor, and control impurities.
Degradation impurities in Gefitinib are the impurities formed during the manufacturing process or storage of the drug product. These impurities can arise from the breakdown of Gefitinib due to various factors such as heat, light, moisture, and pH.
An Analytical method such as RP-HPLC helps detect impurities in Gefitinib.
Gefitinib impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.