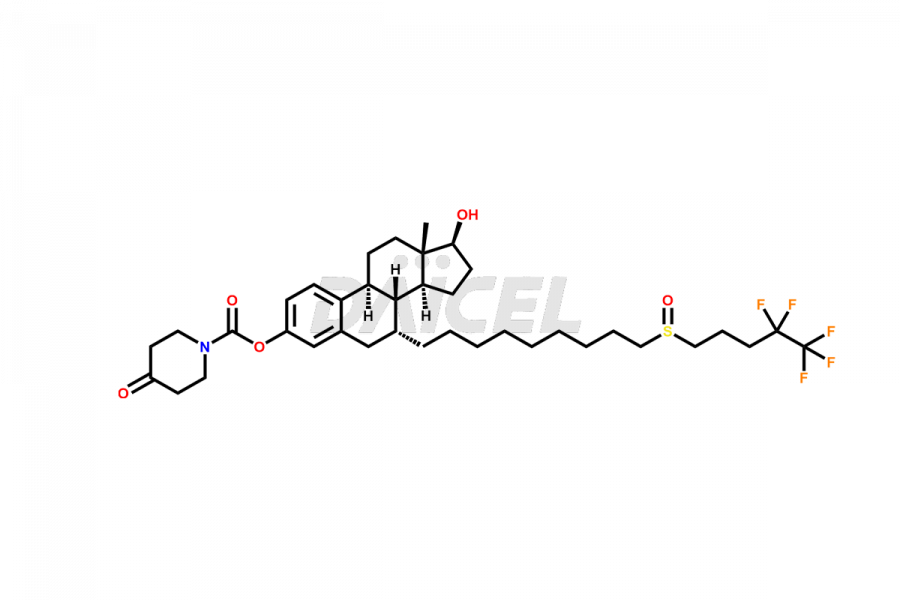
Fulvestrant
General Information
Fulvestrant Impurities and Fulvestrant
Daicel Pharma specializes in synthesizing impurities for Fulvestrant, an active pharmaceutical ingredient. We offer impurities such as AN04035, Fulvestrant R enantiomer, Fulvestrant Related Impurity, and Fulvestrant S enantiomer, which play a vital role in evaluating the purity and safety of Fulvestrant. Daicel Pharma also provides custom synthesis of Fulvestrant impurities to meet specific client needs and offer worldwide delivery options.
Fulvestrant [CAS: 129453-61-8] is a synthetic estrogen receptor antagonist that treats hormone-receptor-positive metastatic breast cancer. It is for estrogen receptor-positive, locally advanced, or metastatic breast cancer cases.
Fulvestrant: Use and Commercial Availability
Fulvestrant, sold under Faslodex, has clinical benefits primarily in postmenopausal women with HR-positive and HER2-negative breast cancer. Fulvestrant treats advanced breast cancer, including cases with large tumors, extensive lymph node metastasis, and invasion into surrounding tissues and organs.
Fulvestrant Structure and Mechanism of Action 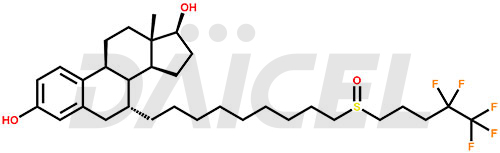
The chemical name of Fulvestrant is (7α,17β)-7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol. Its chemical formula is C32H47F5O3S, and its molecular weight is approximately 606.8 g/mol.
Fulvestrant binds to the estrogen receptors and downregulates the ER protein in human breast cancer cells.
Fulvestrant Impurities and Synthesis
Impurities in Fulvestrant are unintended substances that may be present in the drug product, apart from the desired active ingredient. They can originate from various sources, including raw materials, synthesis processes, or degradation of Fulvestrant over time. Examples of impurities in Fulvestrant can include related compounds, residual solvents, degradation products, or impurities introduced during manufacturing. Effective control and monitoring of impurities in Fulvestrant are essential to ensure the medicine’s quality, safety, and efficacy.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fulvestrant impurity standards like AN04035, Fulvestrant R enantiomer, Fulvestrant Related Impurity, and Fulvestrant S enantiomer. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis1,2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Fulvestrant impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Fazioni, Cristian; Giolito, Andrea, Separation Of Fulvestrant Isomers, WO2007044662A2, April 19, 2007
- Venkata Narasimha Rao, Ganipisetty; Gnanadev, G.; Ravi, Bellam; Dhananjaya, D.; Manoj, P.; Indu, B.; Nadh, R. Venkata, Supercritical fluid (carbon dioxide) based ultra performance convergence chromatography for the separation and determination of fulvestrant diastereomers, Analytical Methods, Volume: 5, Issue: 18, Pages: 4832-4837, 2013
Frequently Asked Questions
Are Fulvestrant impurities continuously monitored during its manufacturing process?
The impurities in Fulvestrant are continuously monitored during its manufacturing process. Quality control measures, including in-process testing and analysis, ensure the ongoing control of impurity levels.
Can Fulvestrant impurities result in variability in its therapeutic response?
Impurities in Fulvestrant have the potential to cause variability in its therapeutic response. They can interfere with the drug's mechanism of action or alter its pharmacokinetics. Their strict control help minimizes such variability to ensure a consistent therapeutic response.
Are there any specific limits or thresholds for impurity levels in Fulvestrant?
Yes, regulatory authorities provide specific limits or thresholds for impurity levels in Fulvestrant. These limits are established based on safety considerations and are included in regulatory guidelines. Manufacturers must adhere to these limits to ensure the quality and safety of Fulvestrant.
How should Fulvestrant impurities be stored in terms of temperature?
The recommendation is to store Fulvestrant impurities at a controlled room temperature, within 2-8 °C (Light sensitive). However, Fulvestrant impurities such as AN04035 are stored at -20 °C in a nitrogen atmosphere.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.