Frovatriptan
General Information
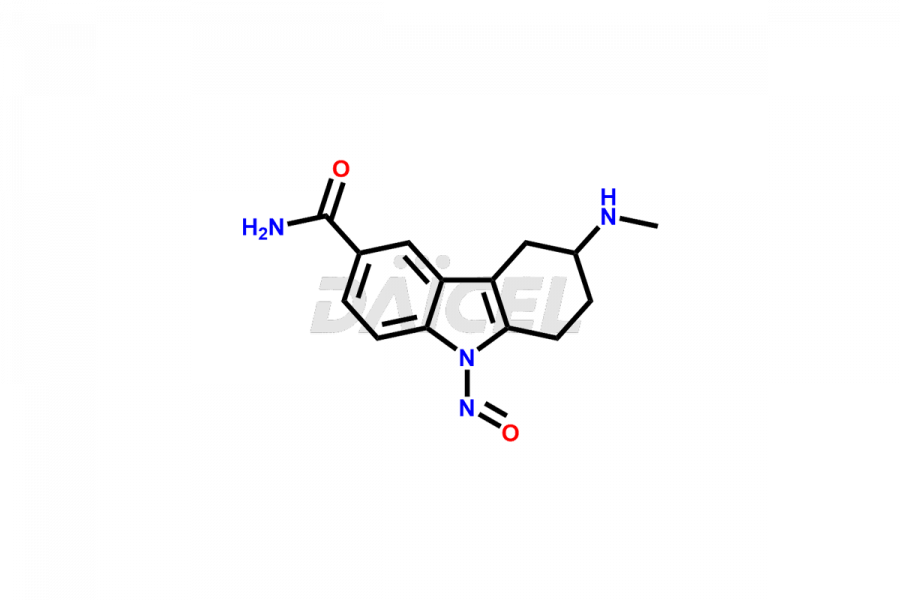
Frovatriptan Impurities and Frovatriptan
Daicel Pharma specializes in synthesizing impurities for Frovatriptan, an active pharmaceutical ingredient. We offer impurities such as 1-(R)-Hydroxy Frovatriptan and 1-(S)-Hydroxy Frovatriptan, which play a vital role in evaluating the purity and safety of Frovatriptan. Daicel Pharma also provides custom synthesis of Frovatriptan impurities to meet specific client needs and offer worldwide delivery options.
Frovatriptan [CAS: 158747-02-5] is a medication, a second-generation member of triptans, and functions as a selective agonist of the serotonin 5-HT1B/D receptor. It has a similar action to sumatriptan, the initial triptan and representative of this class. Frovatriptan gives immediate relief from migraine attacks.
Frovatriptan: Use and Commercial Availability
Frovatriptan, sold under Frova, is a triptan medication for treating migraine headaches, particularly those associated with menstruation. It causes vasoconstriction of the arteries and veins that supply blood to the head. Frovatriptan helps in the short-term prevention of menstrual migraine.
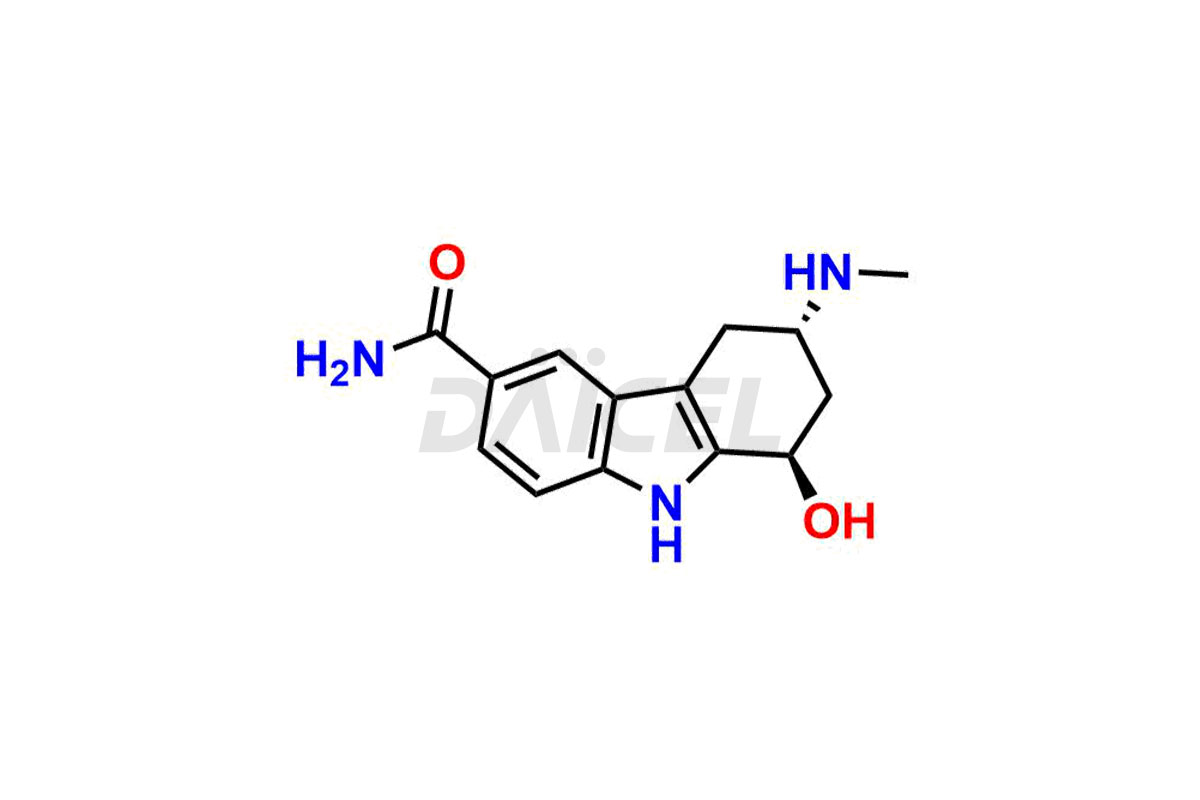
Frovatriptan Structure and Mechanism of Action 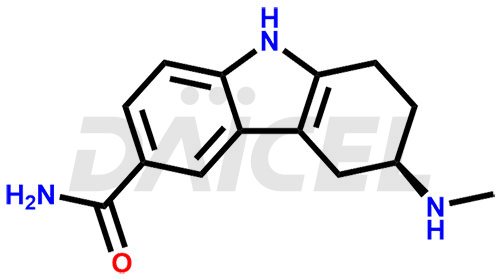
The chemical name of Frovatriptan is (3R)-2,3,4,9-Tetrahydro-3-(methylamino)-1H-carbazole-6-carboxamide. Its chemical formula is C14H17N3O, and its molecular weight is approximately 243.30 g/mol.
Frovatriptan binds with 5-HT1B and 5-HT1D receptors and inhibits excess dilation of the extracerebral and intracranial arteries.
Frovatriptan Impurities and Synthesis
Impurities in Frovatriptan are unintended substances that may be present in the drug product, apart from the intended active ingredient. They can arise from various sources such as raw materials, manufacturing processes1, or degradation of Frovatriptan over time. Examples of impurities in Frovatriptan can include related compounds, residual solvents, degradation products, or impurities introduced during synthesis. Controlling and monitoring impurities in Frovatriptan is crucial to ensure the safety and quality of the medication.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Frovatriptan impurity standards like 1-(R)-Hydroxy Frovatriptan and 1-(S)-Hydroxy Frovatriptan. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we provide additional data like 13C-DEPT. We can synthesize unknown Frovatriptan impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Kitteringham, John; Porter, Roderick A.; Shipton, Mark R.; Vimal, Mythily; Young, Rodney C.; Borrett, Gary T., Process for preparing an enantiomer of a carbazole derivative, Smithkline Beecham P.L.C., United Kingdom, US5618948A, April 8, 1997
- Khan, Muzaffar; Viswanathan, Balaji; Rao, D. Sreenivas; Reddy, Rajasekhar, Chiral separation of Frovatriptan isomers by HPLC using amylose based chiral stationary phase, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences Volume: 846, Issue: 1-2, Pages: 119-123, 2007
Frequently Asked Questions
Are Frovatriptan impurities monitored during its shelf life?
The impurities in Frovatriptan are monitored during its shelf life to ensure the stability and quality of the medication. Stability testing helps assess their presence and levels over the designated shelf-life period. It ensures that impurity levels remain within acceptable limits and do not increase over time.
How are Frovatriptan impurities reported and managed by regulatory authorities?
Regulatory authorities require manufacturers to provide detailed information about impurities in Frovatriptan as part of the drug approval process. It includes the identification, characterization, and control strategies for impurities. Regulatory agencies review and assess the impurity data to ensure compliance with established guidelines and to verify the safety and quality of Frovatriptan.
Which solvent helps in analyzing Frovatriptan impurities?
Water is the solvent used when analyzing many impurities in Frovatriptan.
How should Frovatriptan impurities be stored in terms of temperature?
The recommendation is to store Frovatriptan impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.