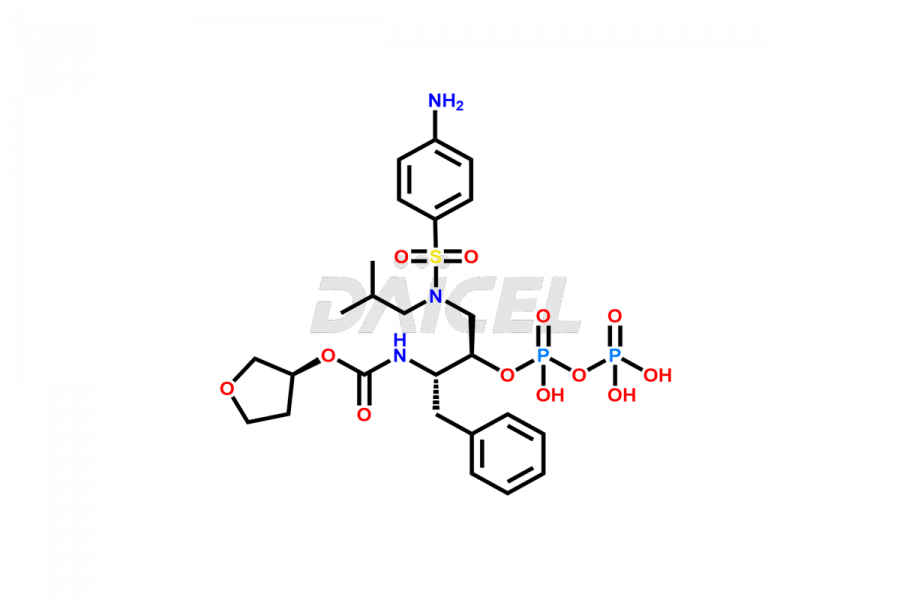
Fosamprenavir
General Information
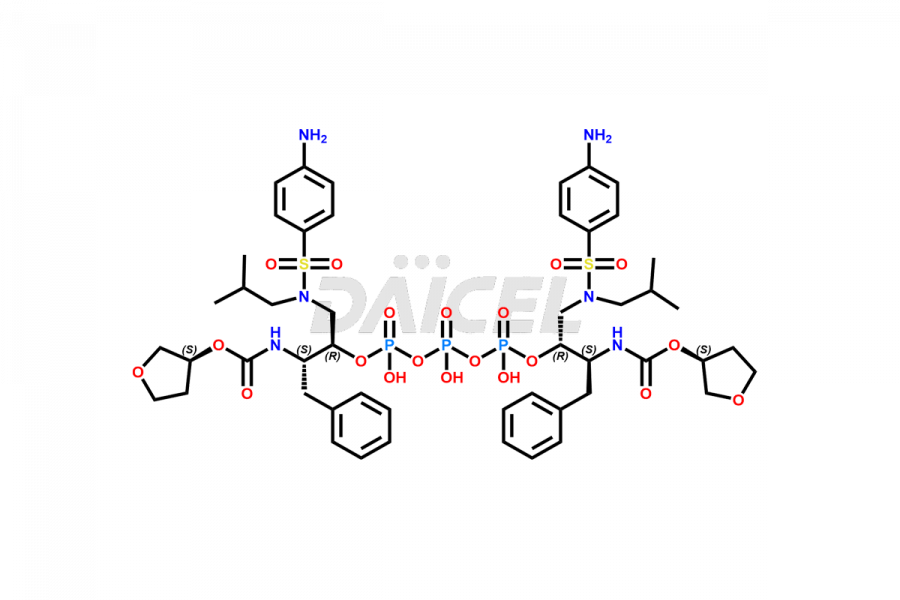
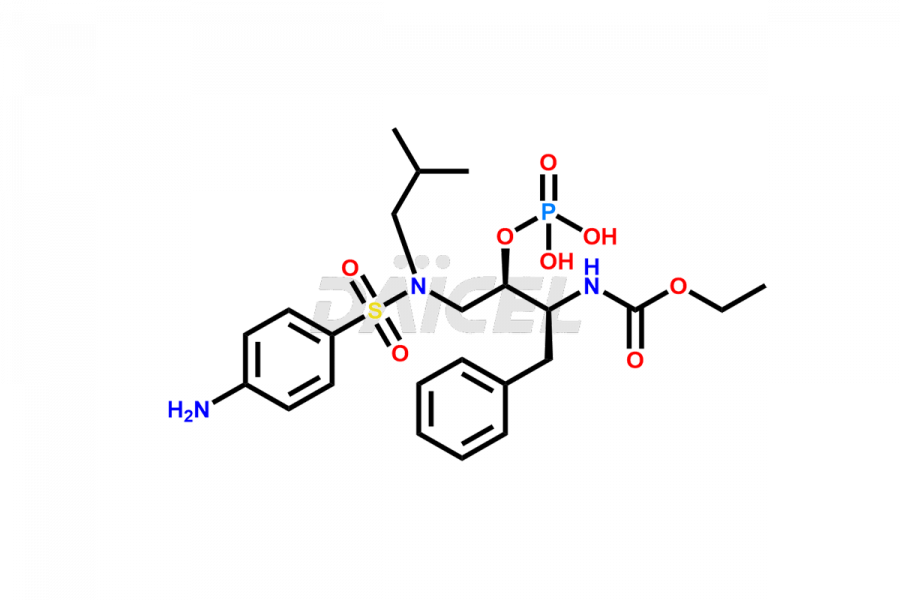
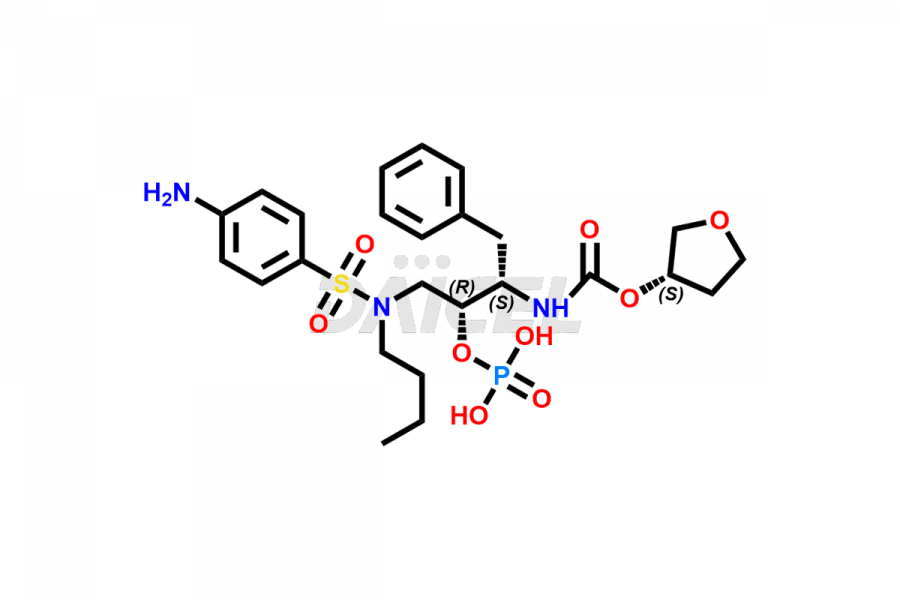
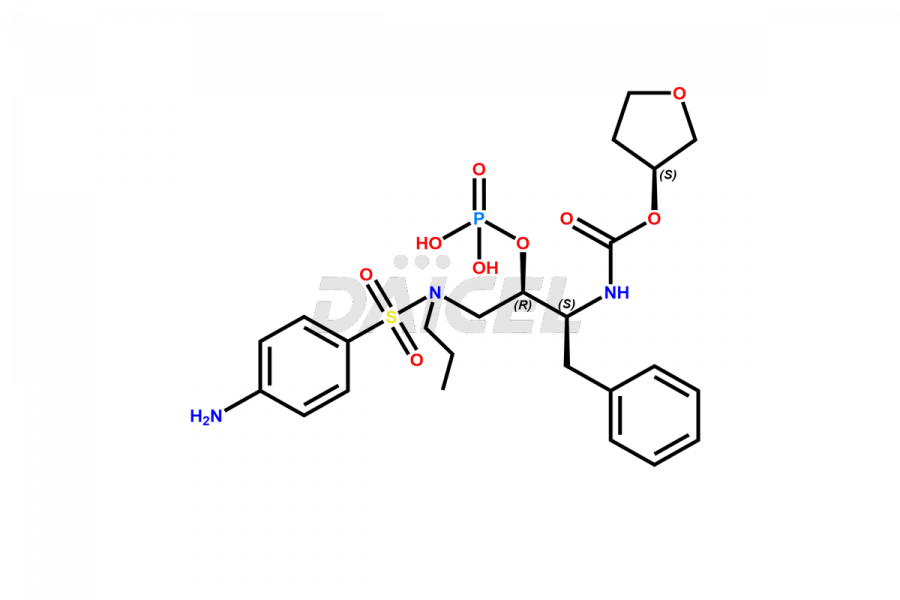
Fosamprenavir Impurities and Fosamprenavir
Daicel Pharma specializes in synthesizes impurities for Fosamprenavir, an active pharmaceutical ingredient. We offer impurities such as Bis Fosamprenavir Triphosphate Impurity, Fosamprenavir Amine Impurity, Fosamprenavir Ethyl Ester Impurity, Fosamprenavir Impurity 2, Fosamprenavir N-Butyl isomer Impurity, Fosamprenavir N-Propyl Homolog Impurity, and Fosamprenavir Pyrophosphate Impurity, which play a vital role in evaluating the purity, and safety of Fosamprenavir. Daicel Pharma also provides custom synthesis of Fosamprenavir impurities to meet specific client needs and offer worldwide delivery options.
Fosamprenavir [CAS: 226700-79-4] is a prodrug of the antiretroviral drug and HIV protease inhibitor, amprenavir. As a prodrug, Fosamprenavir serves as a precursor that undergoes metabolic conversion to its active form. It is functionally related to sulfanilamide compounds. Fosamprenavir is U.S. Food and Drug Administration (FDA) approved for treating HIV infection in adults and children. It is a vital component of antiretroviral therapy used to manage HIV.
Fosamprenavir: Use and Commercial Availability
Fosamprenavir, known by Lexiva, is a protease inhibitor for treating HIV-1 infection. Additionally, Fosamprenavir is for postexposure prophylaxis in individuals exposed to potentially infectious body fluids of an HIV-infected person, particularly in cases with a significant risk of HIV transmission. As a prodrug of amprenavir, Fosamprenavir undergoes conversion to amprenavir within the body. Amprenavir functions by selectively binding and inhibiting the HIV protease.
Fosamprenavir Structure and Mechanism of Action 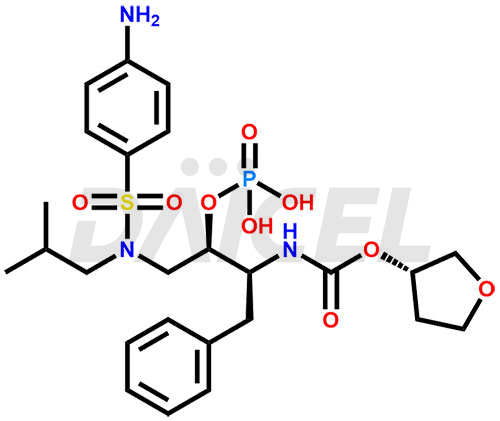
The chemical name of Fosamprenavir is [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-1-(phenylmethyl)-2-(phosphonooxy)propyl]- Carbamic acid C-[(3S)-tetrahydro-3-furanyl] ester. Its chemical formula is C25H36N3O9PS, and its molecular weight is approximately 585.6 g/mol.
Fosamprenavir hydrolyses to amprenavir in the gut epithelium by cellular phosphatases.
Fosamprenavir Impurities and Synthesis
Fosamprenavir, a protease inhibitor used in treating HIV infection, may contain impurities that can arise during the manufacturing process1 or storage conditions. These impurities can include related compounds, degradation products, or residual solvents. The Fosamprenavir impurities can impact its stability, efficacy, and safety. Therefore, stringent quality control measures are implemented during the manufacturing of Fosamprenavir to minimize impurity levels and ensure the purity of the final product. Pharmaceutical manufacturers conduct thorough analysis and monitoring of impurities to comply with regulatory requirements and maintain the quality of Fosamprenavir.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fosamprenavir impurity standards like Bis Fosamprenavir Triphosphate Impurity, Fosamprenavir Amine Impurity, Fosamprenavir Ethyl Ester Impurity, Fosamprenavir Impurity 2, Fosamprenavir N-Butyl isomer Impurity, Fosamprenavir N-Propyl Homolog Impurity, and Fosamprenavir Pyrophosphate Impurity. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Fosamprenavir impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
Frequently Asked Questions
Are Fosamprenavir impurities monitored during clinical trials?
Yes, impurities in Fosamprenavir are monitored during clinical trials to assess their impact on safety and efficacy. Comprehensive analytical testing can identify and quantify impurities. Further, any potential adverse effects associated with impurities need evaluation to ensure patient safety.
Can Fosamprenavir impurities be removed or reduced through purification processes?
Purification processes during the manufacturing of Fosamprenavir minimize impurities. Techniques such as crystallization, filtration, and chromatography help remove or reduce them. These processes contribute to the overall purity and quality of Fosamprenavir.
Do impurity levels in Fosamprenavir influence storage conditions?
Storage conditions can potentially impact impurity levels in Fosamprenavir. Factors such as temperature, humidity, and exposure to light can contribute to the degradation of the medication and the formation of impurities. Proper storage practices, as indicated in the product labeling, help maintain the stability and integrity of Fosamprenavir.
How should Fosamprenavir impurities be stored in terms of temperature?
The recommendation is to store Fosamprenavir impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.