Flurbiprofen
General Information
Flurbiprofen Impurities and Flurbiprofen
Daicel Pharma synthesizes high-quality Flurbiprofen impurities, (R) – Flurbiprofen, and (S) – Flurbiprofen, which is crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Flurbiprofen. Moreover, Daicel Pharma offers custom synthesis of Flurbiprofen impurities and delivers them globally.
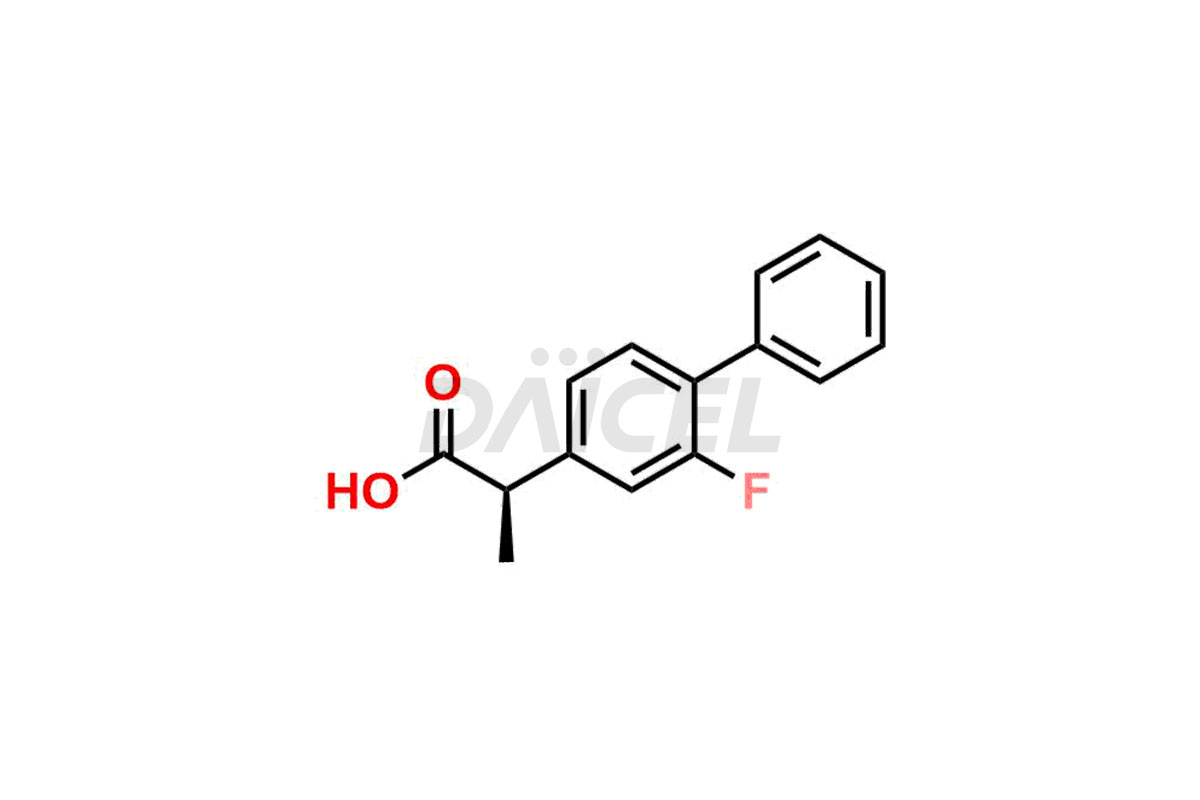
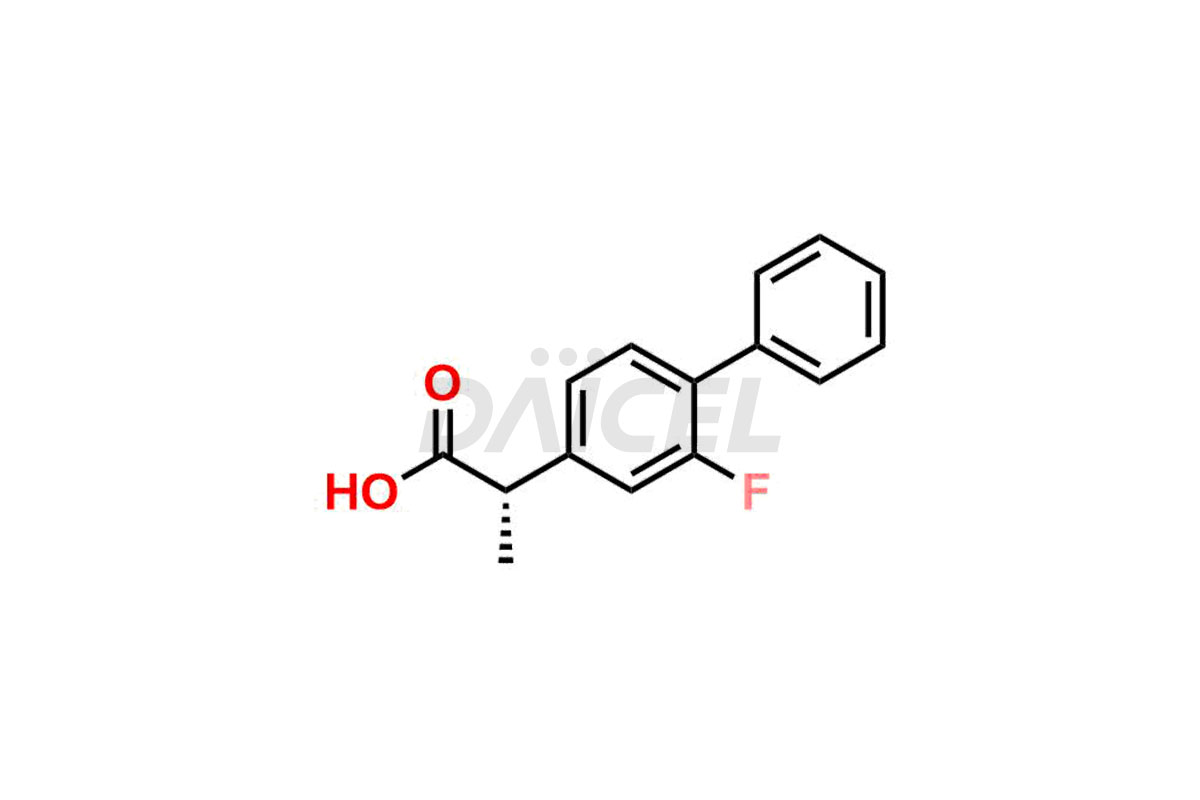
Flurbiprofen [CAS: 5104-49-4] is a propionic acid derivative. It is also a phenylalkanoic acid derivative of non-steroidal anti-inflammatory drug (NSAID). It has analgesic, anti-inflammatory, and antipyretic effects. It relieves mild-to-moderate pain and symptoms of chronic arthritis.
Flurbiprofen: Use and Commercial Availability
Flurbiprofen is used to treat symptoms of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. It can also relieve dysmenorrhea and mild-to-moderate pain associated with inflammation, such as tendonitis, bursitis, or soft tissue trauma. Flurbiprofen can also be applied topically in the eye before surgery to prevent intraoperative miosis. Flurbiprofen is available under different brand names, such as Asaid and Ocufen.
Flurbiprofen Structure and Mechanism of Action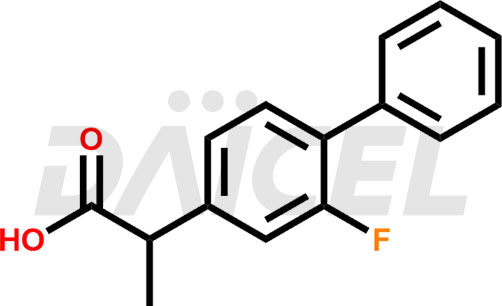
The chemical name of Flurbiprofen is 2-(2-Fluoro-1,1′-biphenyl-4-yl)propanoic acid. Its chemical formula is C15H13FO2, and its molecular weight is approximately 244.26 g/mol.
Though the mechanism of action is unclear, it may be related to prostaglandin synthetase inhibition.
Flurbiprofen Impurities and Synthesis
Flurbiprofen’s efficacy and safety can be affected by impurities. Formation of an impurity generates from synthesis1, raw materials, and storage conditions. Flurbiprofen impurities include isomers, epimers, and degradation products. It is essential to identify and quantify impurities to ensure Flurbiprofen quality.
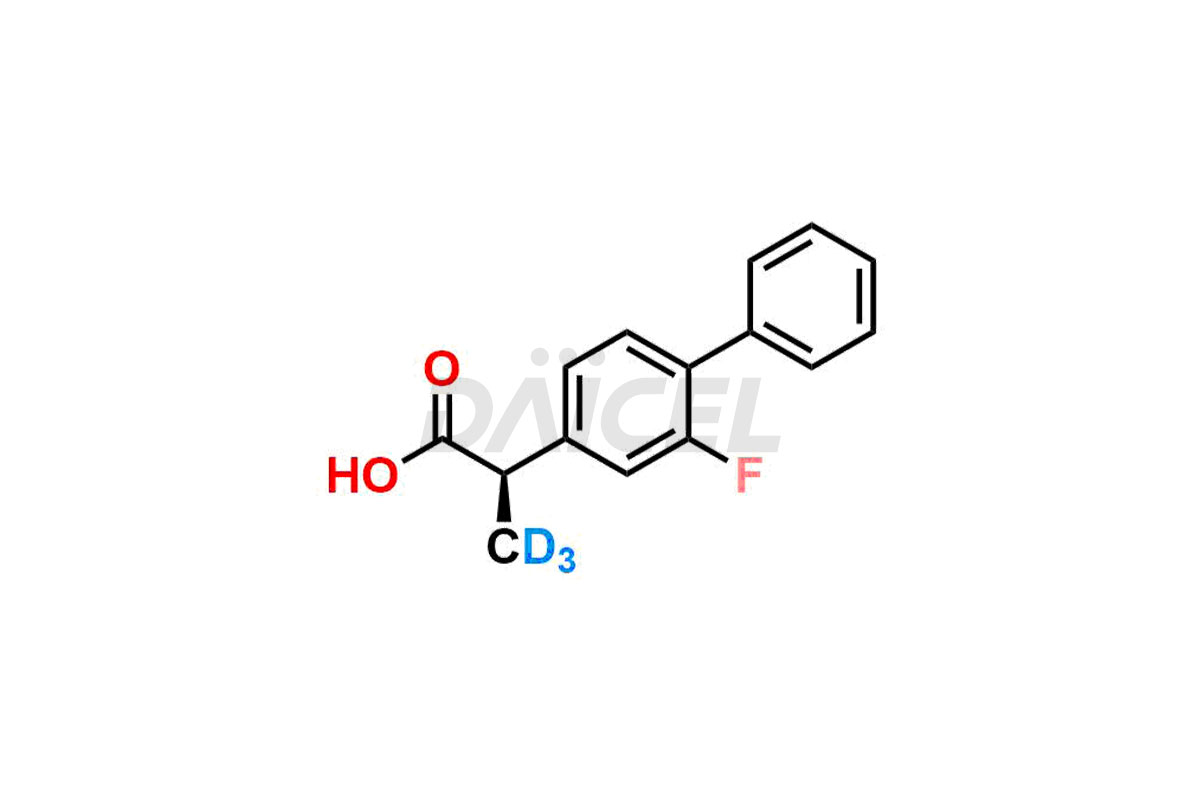
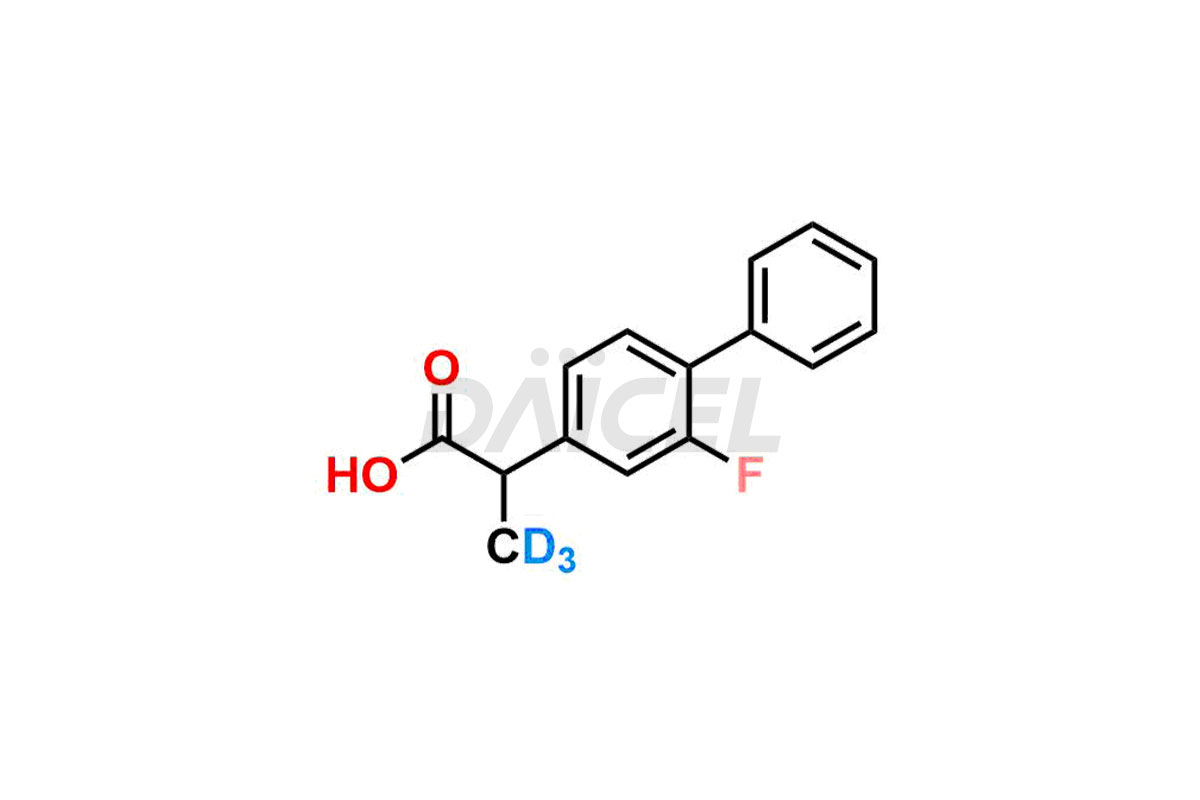
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Flurbiprofen impurity standards, (R) – Flurbiprofen, and (S) – Flurbiprofen. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Flurbiprofen impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Flurbiprofen. Daicel offers (R) – Flurbiprofen-D3, (S) – Flurbiprofen-D3, and Flurbiprofen – D3, which are deuterium-labeled standards of Flurbiprofen for bioanalytical research and BA/BE studies with isotope data in CoA.
References
FAQ's
References
- Adams S, Blancafort A, 2-(Mono-And Difluoro-4-Biphenyl)Propionic Acids, Boots Pure Drug Co., Ltd., Netherlands, US3755427A, August 28, 1973
- Kaiser, David G.; Shaw, S. Robert; Vangiessen, G. J., GLC [gas-liquid chromatography] determination of dl-2-(2-fluoro-4-biphenylyl)propionic acid (flurbiprofen) in plasma, Journal of Pharmaceutical Sciences, Volume: 63, Issue: 4, Pages: 567-70, 1974
Frequently Asked Questions
What is the purpose of synthesizing Flurbiprofen impurities?
Synthesis of Flurbiprofen impurities helps identify and quantify impurities in the drug substance or finished drug product, ensuring their safety and efficacy. Additionally, their synthesis helps in the development and optimization of the manufacturing process of the drug.
How are Flurbiprofen impurities detected and quantified?
The detection and quantification of impurities in Flurbiprofen involve analytical techniques such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS).
Which solvent is for the analysis of Flurbiprofen impurities?
The choice of solvent for analyzing impurities will depend on several factors, such as the chemical nature, the analytical technique used, and the desired sensitivity and selectivity of the analysis. Generally, a polar solvent like methanol helps in analyzing Flurbiprofen impurities.
What is the temperature required to store Flurbiprofen impurities?
Flurbiprofen impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.