LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma specializes in synthesizing impurities for Fluphenazine, an active pharmaceutical ingredient. We offer impurities such as Fluphenazine Impurity-C, Fluphenazine Impurity-D, 7-Hydroxy Fluphenazine, 7-Bromo fluphenazine, Fluphenazine EP Impurity-A, Fluphenazine Impurity-B, and so on, which play a vital role in evaluating the purity, and safety of Fluphenazine. Daicel Pharma also provides custom synthesis of Fluphenazine impurities to meet specific client needs and offer worldwide delivery options.
Fluphenazine [CAS: 69-23-8] is a phenothiazine antipsychotic drug with dopaminergic antagonist properties. It blocks postsynaptic dopamine D2 receptors and reduces the hallucinations associated with schizophrenia. Its uses are similar to chlorpromazine.
Fluphenazine, available under various brand names such as Permitil, Prolixin, Prolixin Decanoate, and Prolixin Enanthate, is a first-generation antipsychotic primarily used for managing symptoms of psychosis in individuals with schizophrenia. It is particularly beneficial for patients who have difficulty tolerating oral medications or when ensuring medication compliance becomes a concern for healthcare providers.
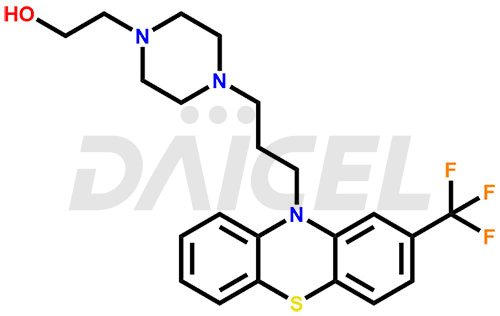
The chemical name of Fluphenazine is 4-[3-[2-(Trifluoromethyl)-10H-phenothiazin-10-yl]propyl]-1-piperazineethanol. Its chemical formula is C22H26F3N3OS, and its molecular weight is approximately 437.5 g/mol.
Fluphenazine blocks dopamine receptors in the limbic system, cortical system, and basal ganglia.
Fluphenazine is a phenothiazine antipsychotic medication that may contain impurities. They can arise during the manufacturing process1 or from storage conditions. Common Fluphenazine impurities include related compounds or degradation products. They may impact the drug’s stability, efficacy, and safety. Therefore, pharmaceutical manufacturers need to monitor and control impurity levels to ensure the quality of Fluphenazine products.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fluphenazine impurity standards like Fluphenazine Impurity-C, Fluphenazine Impurity-D, 7-Hydroxy Fluphenazine, 7-Bromo fluphenazine, Fluphenazine EP Impurity-A, Fluphenazine Impurity-B, and so on. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we provide additional data like 13C-DEPT. We can synthesize unknown Fluphenazine impurities, degradation products and labeled compounds to assess the effectiveness of generic Fluphenazine. For bio-analytical research, including BA/BE studies, we offer Fluphenazine-D6 hydrochloride, a deuterated-labeled compound of Fluphenazine. Each delivery has a comprehensive characterization report.
Pharmaceutical manufacturers of Fluphenazine implement rigorous quality control measures to monitor and control impurity levels. These measures include using appropriate manufacturing processes, conducting thorough testing and analysis, and adhering to regulatory guidelines and specifications for impurity limits.
Impurities in Fluphenazine can arise from various sources, including raw materials, reagents during synthesis, degradation of the API over time, or by-products formed during the manufacturing process. Manufacturers need to identify and address these potential sources to maintain the purity of Fluphenazine.
Impurities in Fluphenazine are regularly tested and monitored throughout the manufacturing process and the product's shelf life. Manufacturers conduct routine analysis and stability studies to assess impurity levels and ensure compliance with regulatory requirements. These tests help maintain the quality and integrity of Fluphenazine.
The recommendation is to store Fluphenazine impurities at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.