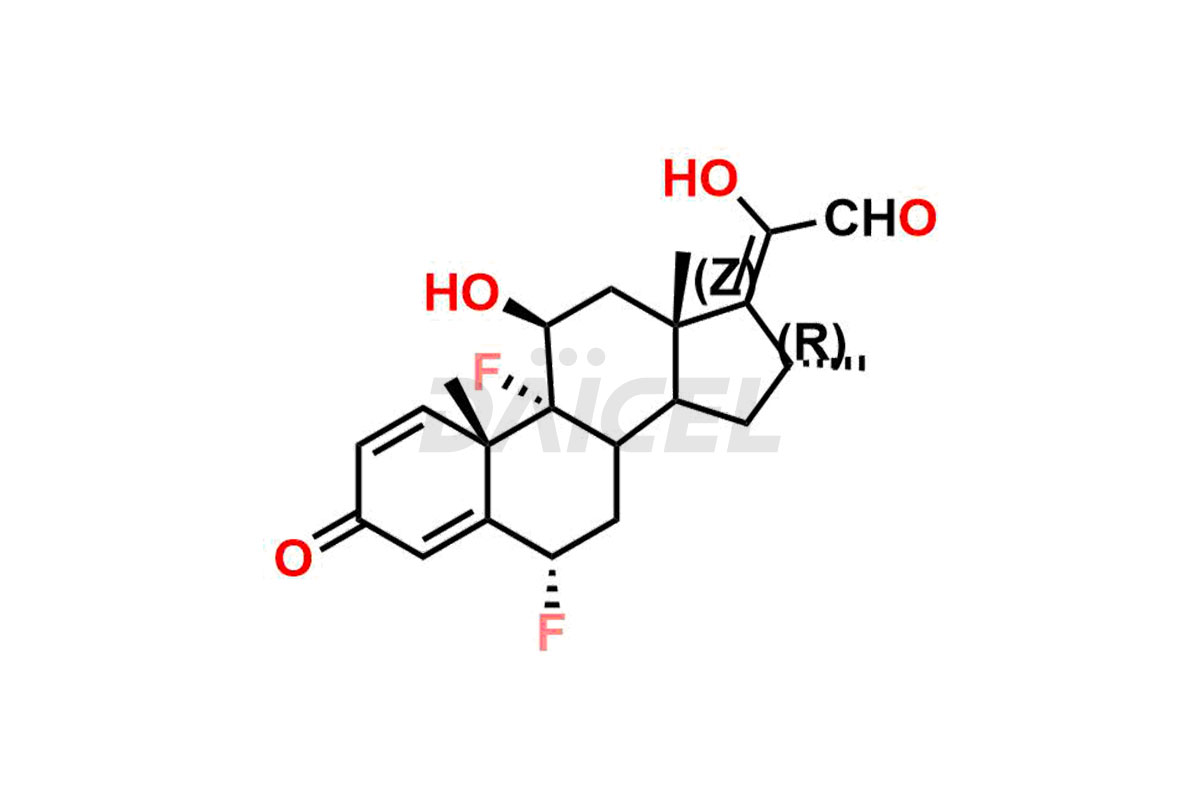
Flumethasone
General Information
Flumethasone Impurities and Flumethasone
Daicel Pharma specializes in synthesizing impurities for Flumethasone, an active pharmaceutical ingredient. We offer impurities such as 17-Keto Flumethasone and Flumethasone impurity 7, which play a vital role in evaluating Flumethasone purity and safety. Daicel Pharma also provides custom synthesis of Flumethasone impurities to meet specific client needs and offer worldwide delivery options.
Flumethasone [CAS: 2135-17-3], a fluorinated steroid derived from a hydride of a pregnane, functions as a glucocorticoid and an anti-inflammatory medication. It is a veterinary drug known for its anti-inflammatory properties in veterinary practice.
Flumethasone: Use and Commercial Availability
Flumethasone, available under the brand name Locorten, belongs to the class of corticosteroids and treats various inflammatory and immune-mediated diseases. Its use is in replacement, anti-inflammatory, and immunosuppressive therapies. Flumethasone treats inflammatory conditions, particularly musculoskeletal disorders. Flumethasone has proven effective in managing recurrent airway obstruction (RAO) in horses. In cattle, corticosteroids help in the treatment of ketosis.
Flumethasone Structure and Mechanism of Action 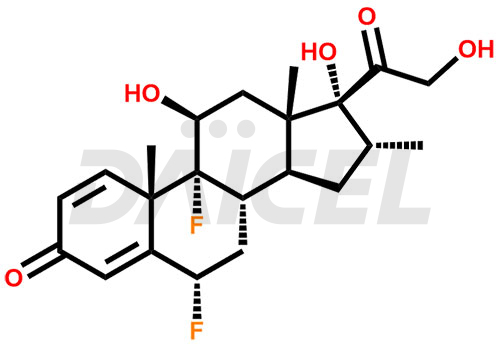
The chemical name of Flumethasone is (6α,11β,16α)-6,9-Difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione. Its chemical formula is C22H28F2O5, and its molecular weight is approximately 410.5 g/mol.
Flumethasone inhibits arachidonic acid, and it decreases lymphatic system functions.
Flumethasone Impurities and Synthesis
Flumethasone impurities are undesired substances that form during the synthesis1, storage, or usage of Flumethasone. They can arise due to various factors, including incomplete reactions, side reactions, degradation, or impure starting materials. The analysis and control of Flumethasone impurities are crucial to ensure Flumethasone purity, safety, and efficacy. Sophisticated analytical techniques, such as liquid chromatography (LC) with mass spectrometry (MS), help identify and quantify these impurities. Strict quality control measures and adherence to regulatory guidelines help minimize impurities and maintain the highest possible quality standards.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Flumethasone impurity standards like 17-Keto Flumethasone and Flumethasone impurity 7. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. We can synthesize unknown Flumethasone impurities or degradation products to assess the effectiveness of generic Flumethasone. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Improvements in or relating to steroids and the manufacture thereof, Upjohn Co., GB902292A, August 1, 1962
- Brambilla, G.; Buiarelli, F.; Cartoni, G. P.; Coccioli, F.; Colamonici, C.; Fagiolo, A.; Giannini, C.; Neri, B., Determination of Flumethasone in calf urine and serum by liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 755, Issue: 1-2, Pages: 265-278, 2001
Frequently Asked Questions
How are Flumethasone impurities identified?
Flumethasone impurities are identified through rigorous analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography (LC), and mass spectrometry (MS). These methods allow for the separation, detection, and structural elucidation of impurities in Flumethasone samples.
How frequently are Flumethasone impurities monitored and tested?
Flumethasone impurities are monitored and tested throughout manufacturing, including during raw material inspection, in-process controls, and final product testing. Regular monitoring and testing ensure compliance with quality standards and verify the absence of harmful impurities.
How are Flumethasone impurities controlled in the storage and stability of the medication?
Flumethasone impurities' control is through appropriate storage conditions and stability testing. It helps identify any impurity formation that may occur over time, and manufacturers establish expiration dates and storage recommendations that ensure the integrity and quality of the medication throughout its shelf life.
How should Flumethasone impurities be stored in terms of temperature?
For proper storage of Flumethasone impurities, it is advisable to keep them at a controlled room temperature, from 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.