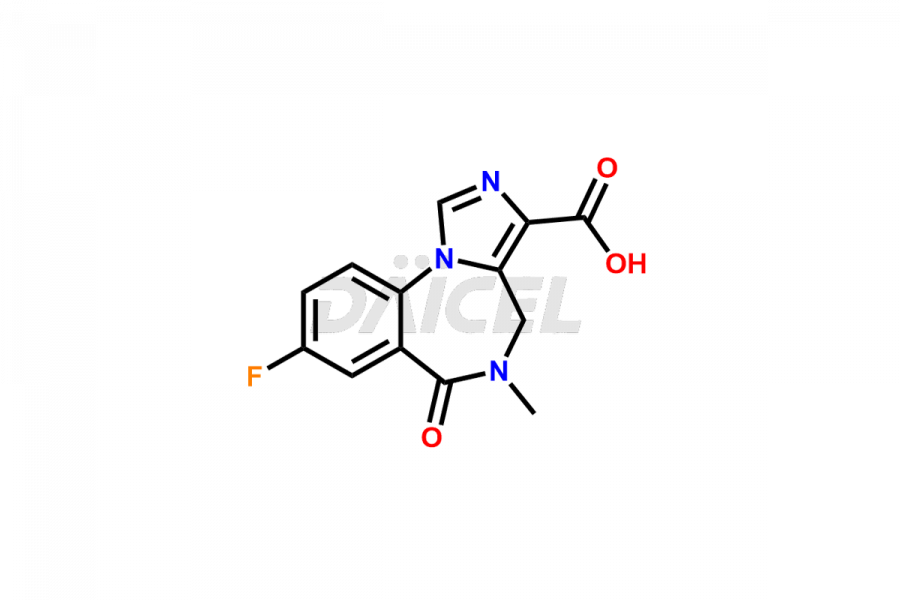
Flumazenil
General Information
Flumazenil Impurities and Flumazenil
Daicel Pharma specializes in synthesizing impurities for Flumazenil, an active pharmaceutical ingredient. We offer crucial impurities such as Flumazenil EP Impurity-B and Flumazenil related compound A, which play a vital role in evaluating Flumazenil purity and safety. Daicel Pharma also provides custom synthesis of Flumazenil impurities to meet specific client needs, and we offer worldwide delivery options.
Flumazenil [CAS: 78755-81-4] is a imidazo-benzodiazepine derivative that functions as a powerful antagonist of benzodiazepine receptors. It effectively counteracts the sedative effects and other actions caused by benzodiazepines, making it a valuable antidote for benzodiazepine overdoses. Acting as an antagonist to GABA, Flumazenil reverses the effects of benzodiazepines and serves as an antidote for cases of benzodiazepine poisoning.
Flumazenil: Use and Commercial Availability
Flumazenil, available under Romazicon, is a benzodiazepine antagonist with approved clinical uses as a reversal agent for benzodiazepine overdose and postoperative sedation caused by benzodiazepine anesthetics. It is for the complete or partial reversal of benzodiazepine-induced sedation in conscious and general anesthesia in adults and pediatric populations.
Flumazenil Structure and Mechanism of Action 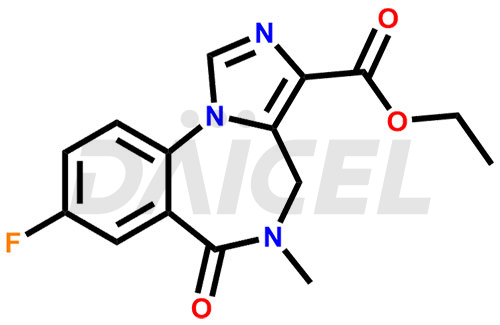
The chemical name of Flumazenil is 8-fluoro-5,6-dihydro-5-methyl-6-oxo- 4H-Imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester. Its chemical formula is C15H14FN3O3, and its molecular weight is approximately 303.29 g/mol.
Flumazenil antagonizes the benzodiazepine actions in the central nervous system. It prevents activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex.
Flumazenil Impurities and Synthesis
Flumazenil impurities can arise from chemical reactions, such as oxidation or hydrolysis, as well as from residual solvents or starting materials. These impurities can potentially affect the stability, efficacy, and safety of Flumazenil1. Rigorous analytical techniques, including high-performance liquid chromatography (HPLC) and mass spectrometry (MS), help identify and quantify impurities within Flumazenil formulations. Through meticulous analysis and control, pharmaceutical manufacturers can ensure that Flumazenil meets stringent quality standards and regulatory requirements and ultimately provide safe and effective medication for patients.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Flumazenil impurity standards like Flumazenil EP Impurity-B and Flumazenil related compound A. We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we provide additional data like 13C-DEPT. We can synthesize unknown Flumazenil impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Mohler, Hanns; Hunkeler, Walter; Polc, Petar; Haefely, Willy; Pieri, Lorenzo; Kyburz, Emilio; Gerecke, Max, Imidazodiazepine Derivatives, Process And Intermediates For Their Preparation, Medicaments Containing Them And Their Therapeutic Application, Hoffmann-La Roche, F., und Co. A.-G., Switzerland, EP27214B1, May 16, 1984
- Bun, H.; Duplan, V.; Crevat-Pisano, P.; Llurens, M.; Durand, A., Rapid determination of the benzodiazepine antagonist flumazenil, Ro 15-1788, by high performance liquid chromatography, Biomedical Chromatography, Volume: 3, Issue: 6, Pages: 269-71, 1989
Frequently Asked Questions
Why is analysis and control of Flumazenil impurities essential?
Analysis and control of Flumazenil impurities are crucial to ensure the medication's safety, efficacy, and quality. They can potentially affect the stability and therapeutic activity or cause adverse effects. Therefore, stringent control measures are necessary to maintain the purity and compliance of Flumazenil.
How are Flumazenil impurities controlled during manufacturing?
Manufacturing processes for Flumazenil involve strict quality control measures to minimize impurity formation. They include proper raw material selection and handling, optimizing reaction conditions, and implementing purification techniques to remove impurities. Regular monitoring and testing ensure compliance with quality standards.
Are Flumazenil impurities harmful?
Some Flumazenil impurities may have the potential to be harmful if present in significant amounts or if they possess toxic properties. So it is necessary to control and minimize Flumazenil's level of impurities.
How should Flumazenil impurities be stored in terms of temperature?
For proper storage of Flumazenil impurities, it is advisable to keep them at a controlled room temperature, from 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.