Fluconazole
General Information
Fluconazole Impurities and Fluconazole
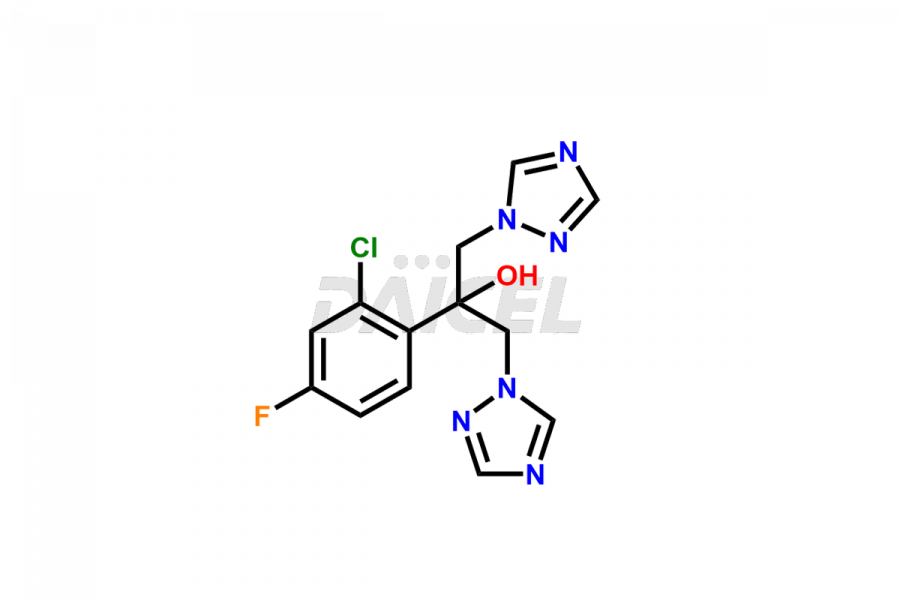
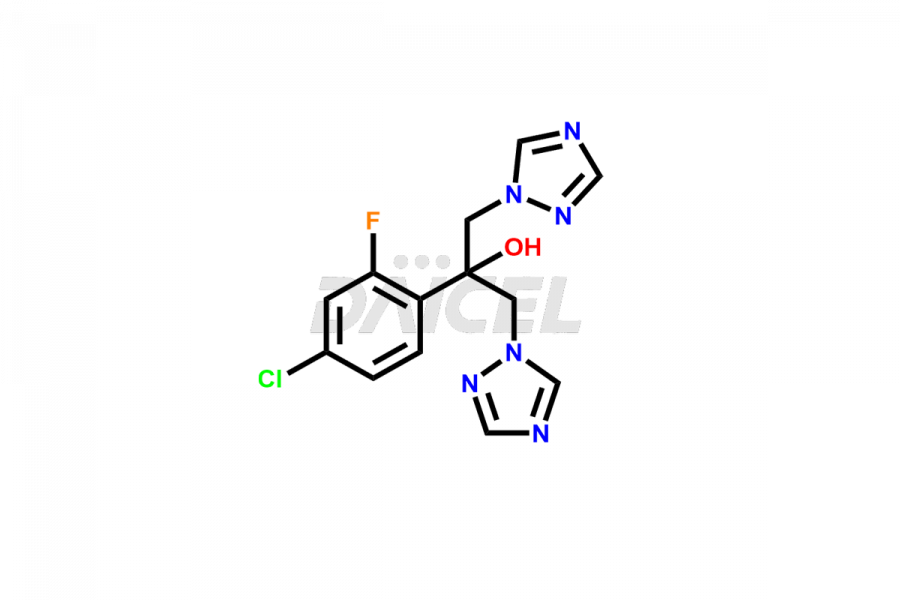
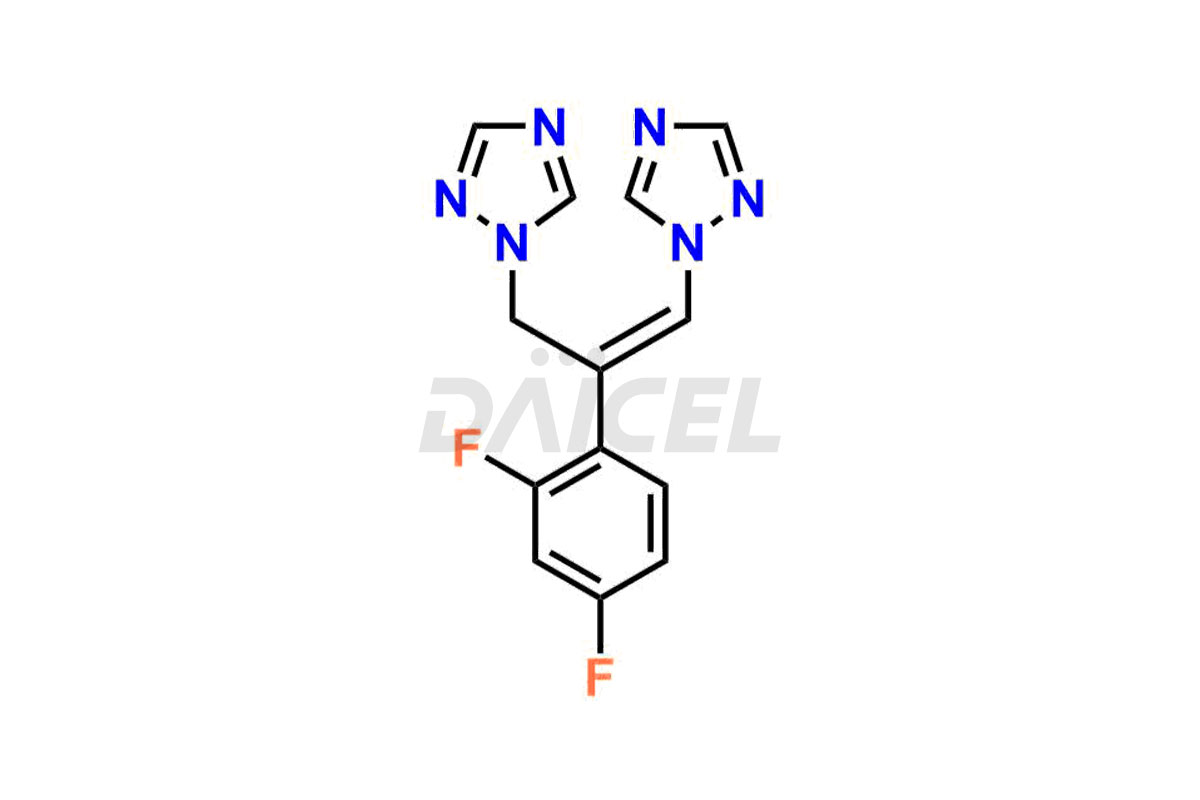
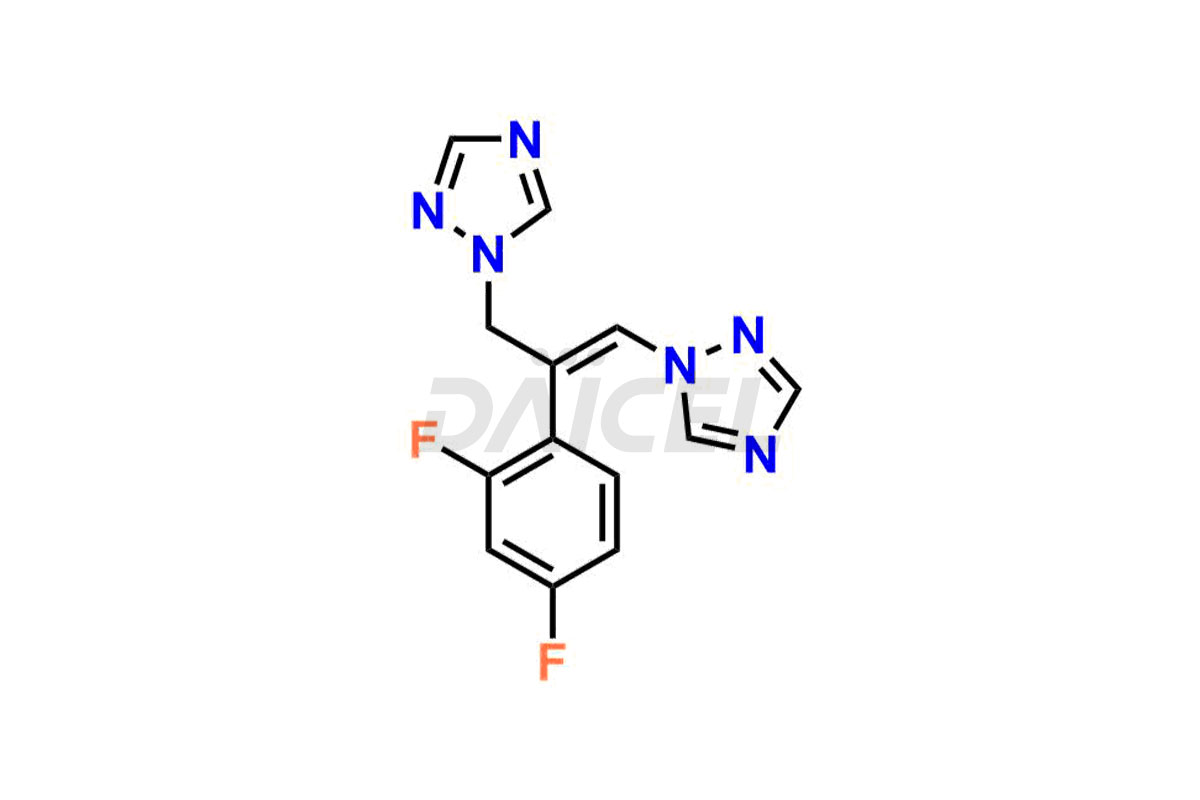
Daicel Pharma specializes in synthesizing impurities for Fluconazole, an active pharmaceutical ingredient. We offer crucial impurities such as 2-(2- Chloro-4-fluorophenyl)-1,3-bis-[1,2,4]triazole-1-yl-propan-2-ol(chloro fluoro impurity), 2-(4-chloro-2-fluorophenyl)-1,3-di(1H-1,2,4-triazol-1-yl)propan-2-ol, Fluconazole Related Compound # 4 (FN RC4), and Fluconazole Related Compound # 6, (FN RC6), which play a vital role in evaluating the purity, and safety of Fluconazole. Daicel Pharma also provides custom synthesis of Fluconazole impurities to meet specific client needs, and we offer worldwide delivery options.
Fluconazole [CAS: 86386-73-4] is an antifungal drug of the triazole class. It treats various fungal infections, including mucosal candidiasis, systemic candidiasis, coccidioidomycosis, and cryptococcosis. Acting as a fungistatic agent, Fluconazole inhibits the growth of fungi. It manages oropharyngeal candidiasis and cryptococcal meningitis in individuals with AIDS.
Fluconazole: Use and Commercial Availability
Fluconazole, or Diflucan, is an antifungal medication for various fungal infections affecting different tissues. It is US FDA-approved for conditions such as vaginal candidiasis, oropharyngeal and esophageal candidiasis, systemic Candida infections (including candidemia and disseminated candidiasis), peritonitis, pneumonia, and cryptococcal meningitis. Additionally, it serves as a prophylactic measure in bone marrow transplantation patients undergoing cytotoxic chemotherapy or radiation therapy.
Fluconazole Structure and Mechanism of Action 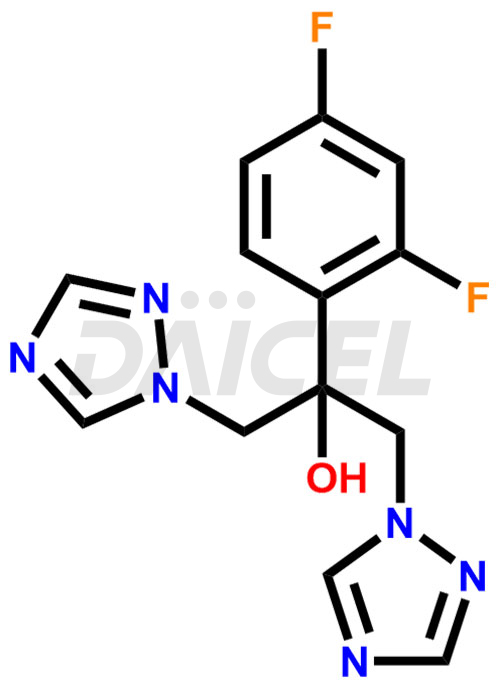
The chemical name of Fluconazole is α-(2,4-Difluorophenyl)-α-(1H-1,2,4-triazol-1-ylmethyl)-1H-1,2,4-triazole-1-ethanol. Its chemical formula is C13H12F2N6O, and its molecular weight is approximately 306.27 g/mol.
Fluconazole prevents fungal cytochrome P-450 sterol C-14 alpha demethylation, causing loss of fungal sterols and fungistatic activity.
Fluconazole Impurities and Synthesis
Impurities found in Fluconazole are unintended substances that can coexist with the active pharmaceutical ingredient. They can originate from various sources, including the manufacturing process1, degradation, or interactions with other components. Rigorous analytical methods, such as chromatography and spectroscopy, help identify, characterize, and quantify these impurities. Strict quality control measures are implemented during production to minimize impurity formation and ensure the purity and safety of Fluconazole. Regulatory guidelines set limits and specifications for acceptable levels of impurities in Fluconazole products, and continuous monitoring and testing uphold the quality and efficacy of Fluconazole as an antifungal agent.
Daicel Pharma, in adherence to cGMP standards, operates an analytical facility where we prepare Fluconazole impurity standards like 2-(2- Chloro-4-fluorophenyl)-1,3-bis-[1,2,4]triazole-1-yl-propane-2-ol(chloro fluoro impurity), 2-(4-chloro-2-fluorophenyl)-1,3-di(1H-1,2,4-triazol-1-yl)propan-2-ol, Fluconazole Related Compound # 4 (FN RC4), and Fluconazole Related Compound # 6, (FN RC6). We offer a comprehensive Certificate of Analysis (CoA) for these impurities, providing a detailed characterization report. The CoA includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we provide additional data like 13C-DEPT. We can synthesize unknown Fluconazole impurities or degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Richardson, Kenneth, Antifungal agents, Processes for their preparation, and pharmaceutical compositions containing them, Pfizer Ltd., United Kingdom, EP69442B1, February 20, 1985
- Kim, Eun J.; Lee, Hye S.; Zee, Ok P.; Lee, Sung T., A sensitive and simplified HPLC analysis for the determination of fluconazole in human plasma, Archives of Pharmacal Research, Volume: 11, Issue: 3, Pages: 250-2, 1988
Frequently Asked Questions
How are Fluconazole impurities controlled during storage and distribution?
Proper storage conditions, such as temperature and humidity control, are essential to prevent the formation of impurities during the storage and distribution of Fluconazole. Good storage practices help maintain the integrity and quality of the product.
Can Fluconazole impurities interact with other medications?
Some impurities in Fluconazole may have the potential to interact with other medications, leading to drug-drug interactions. It is vital to consider their presence when prescribing Felodipine with other drugs.
Which solvent helps in analyzing Fluconazole impurities?
Methanol or Acetonitrile are the commonly used solvents when analyzing many impurities in Fluconazole.
How should Fluconazole impurities be stored in terms of temperature?
The recommended temperature to store Fluconazole impurities is at a controlled room temperature, within 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.