Favipiravir
General Information
Favipiravir Impurities and Favipiravir
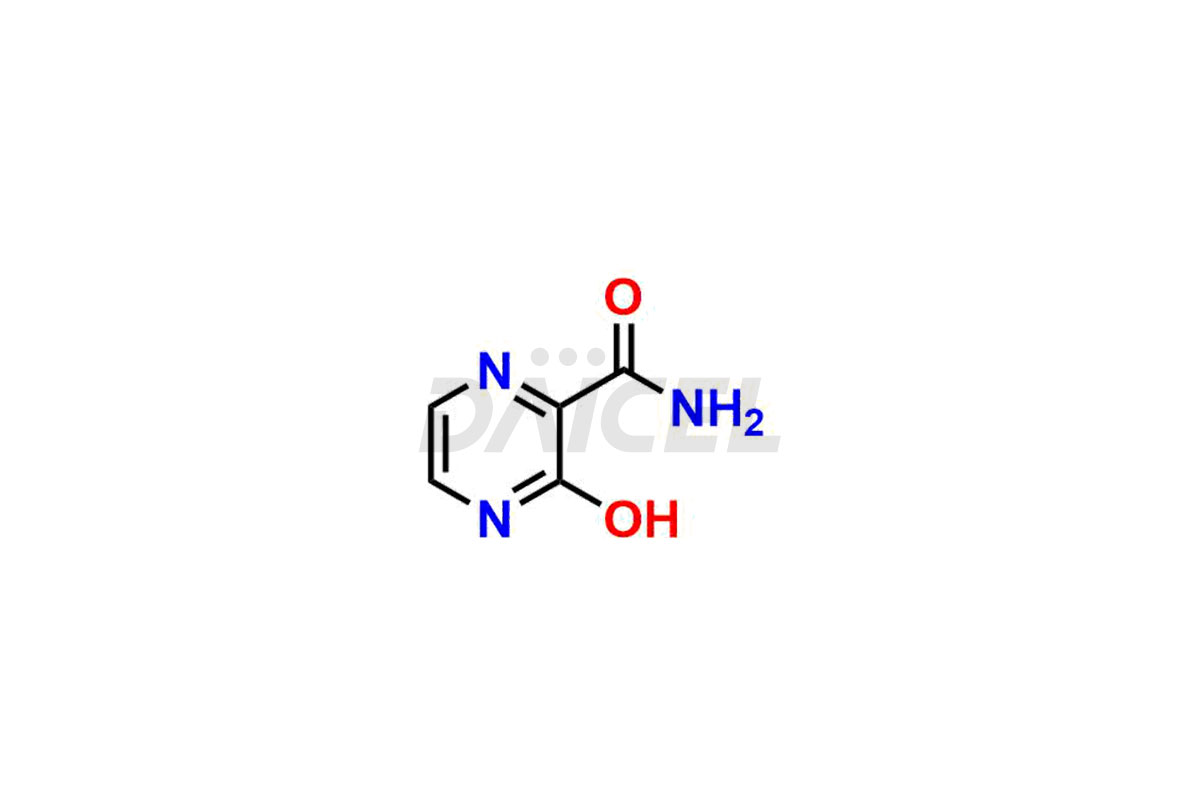
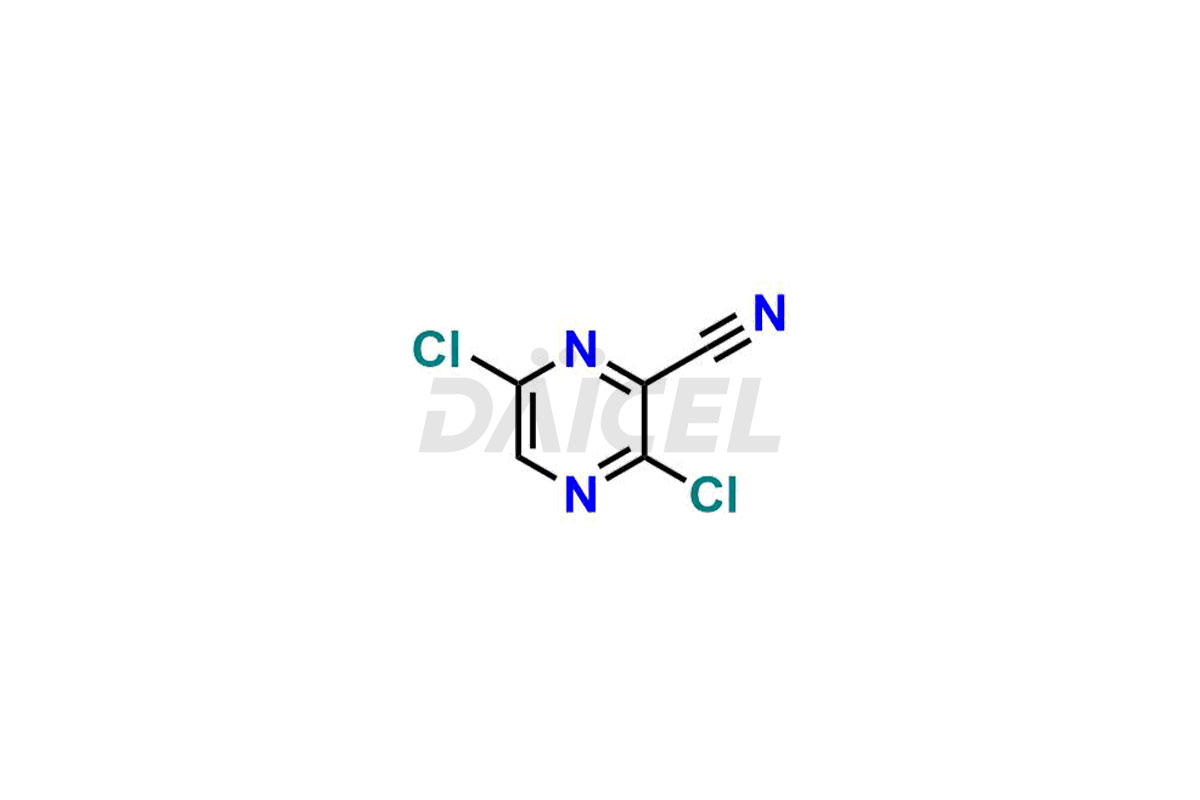
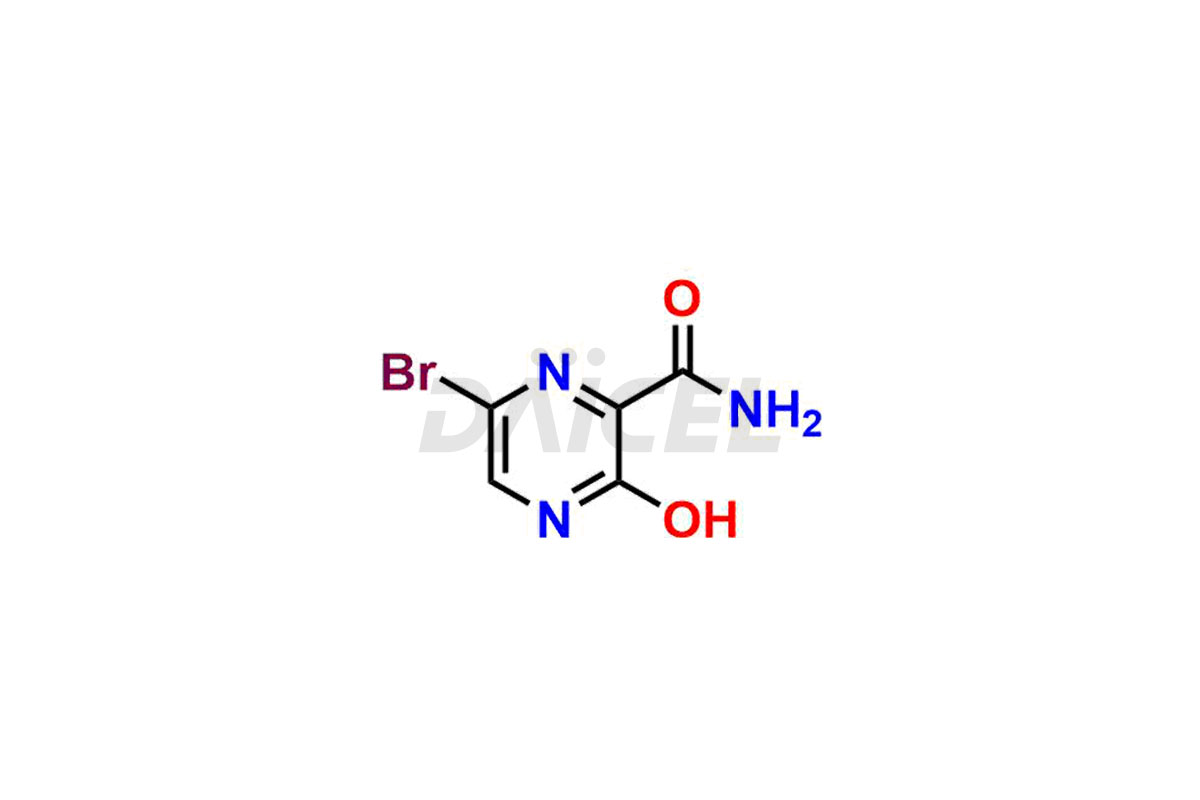
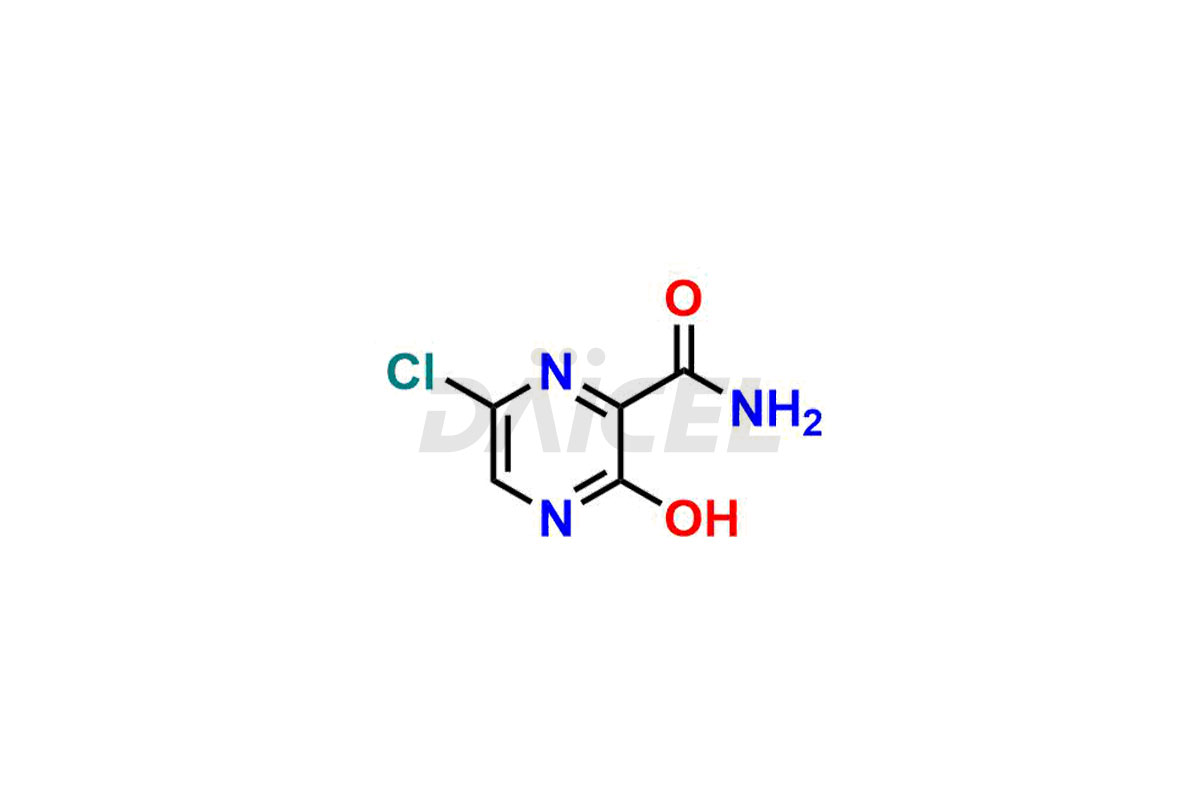
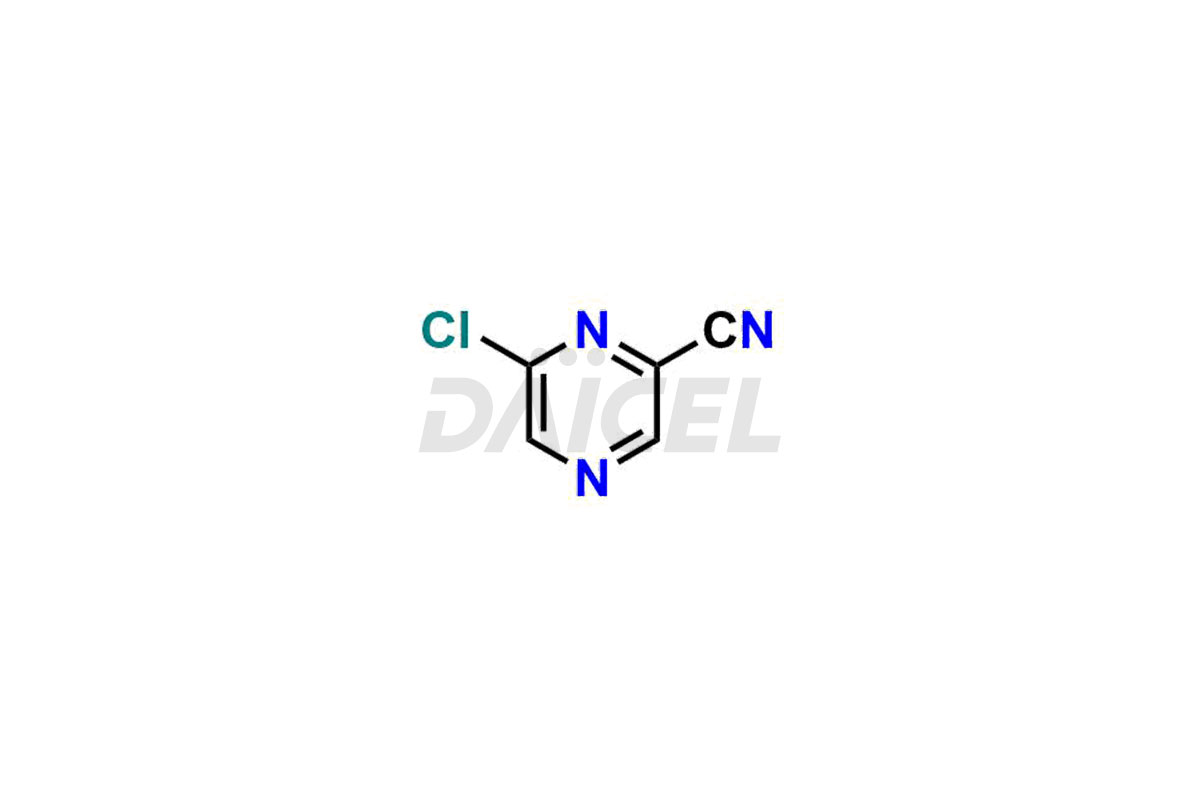
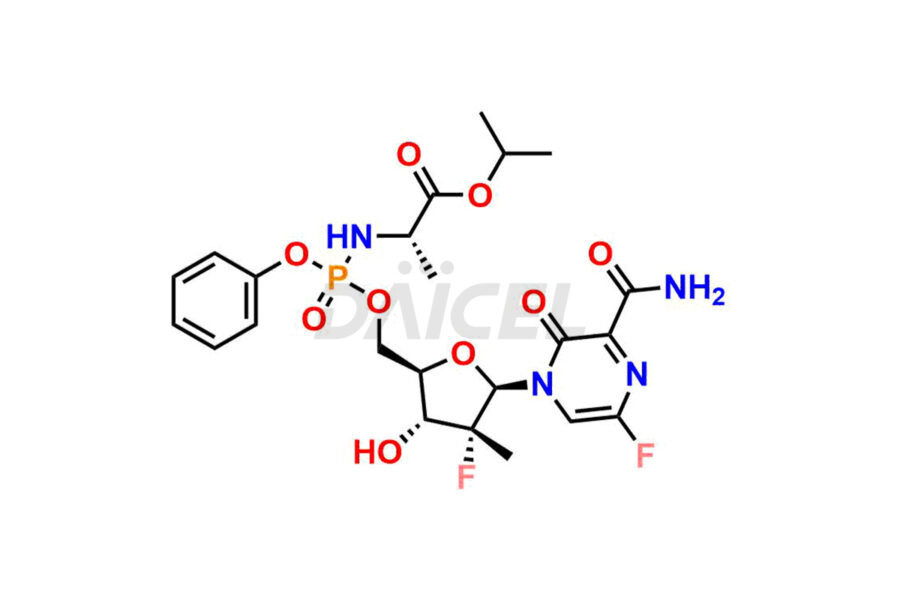
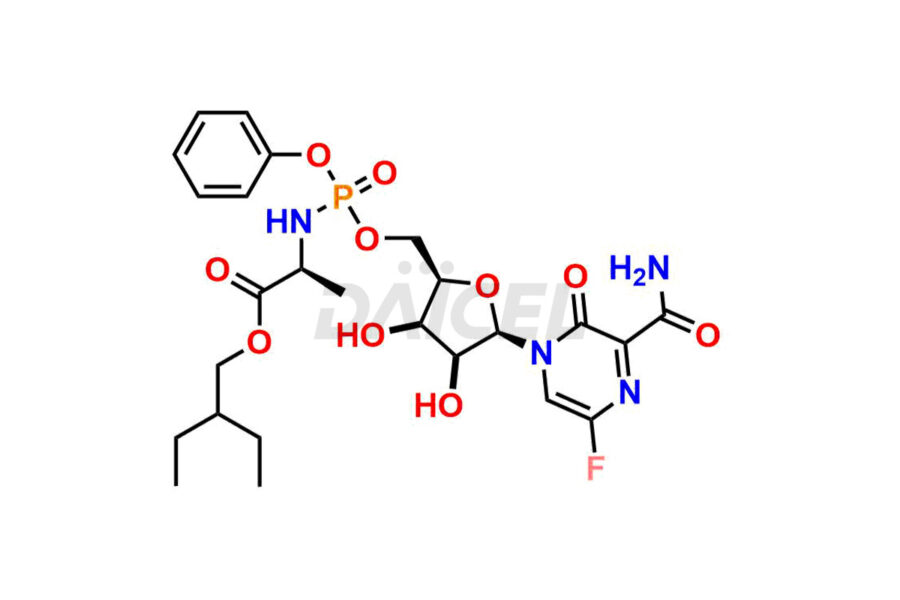
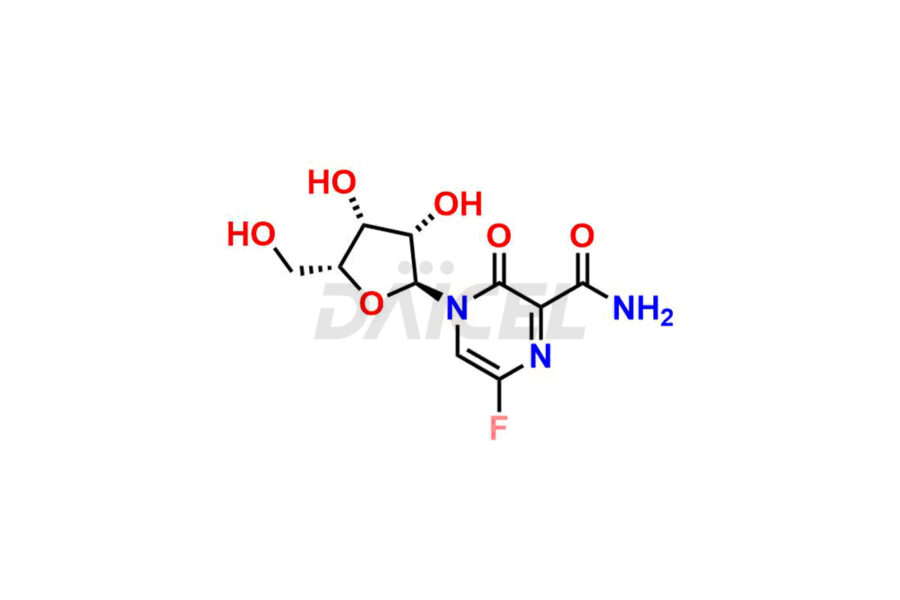
Daicel Pharma offers high-quality impurities for Favipiravir, an active pharmaceutical ingredient. These impurities, including 3-hydroxypyrazine-2-carboxamide, 3,6-dichloropyrazine-2-carbonitrile, 6-bromo-3-hydroxypyrazine-2-carboxamide, 6-Chloro-3-hydroxypyrazine-2-carboxamide, 6-Chloroypyrazine-2-carbonitrile, Favipiravir fluoro Ribofuranose, Favipiravir Ribofuranose, and Favipiravir Ribofuranose metabolite, play a vital role in assessing the purity, reliability, and safety of Favipiravir. Daicel Pharma also offers a customized synthesis of Favipiravir impurities to cater to client requirements, with worldwide delivery options available.
Favipiravir [CAS: 259793-96-9] is an antiviral drug that is a pyrazine carboxamide derivative. It exhibits activity against RNA viruses by selectively inhibiting the RNA-dependent RNA polymerase of RNA viruses. Favipiravir treats influenza and is an antiviral agent and an RNA-directed RNA polymerase inhibitor. Furthermore, it also helps in combating coronaviruses.
Favipiravir: Use and Commercial Availability
Favipiravir, known by the brand name Avigan, was initially approved in Japan in 2014 for treating cases of influenza that were unresponsive to conventional therapies. Its effectiveness in targeting various influenza strains has prompted investigations into its potential use against other viral infections, including Ebola and COVID-19. Favipiravir acts as a purine analog, substituting guanine or adenine during viral replication, thereby hindering the replication process. It can treat life-threatening infections such as Ebola, Lassa fever, and rabies, establishing its therapeutic value in these diseases.
Favipiravir Structure and Mechanism of Action 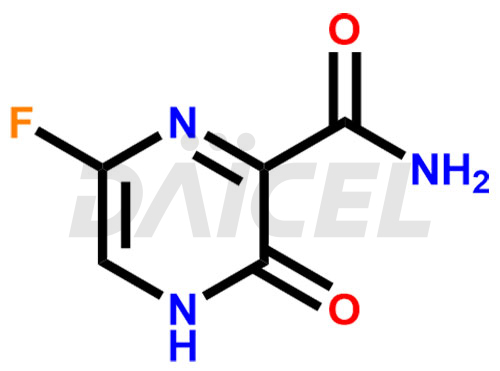
The chemical name of Favipiravir is 6-Fluoro-3,4-dihydro-3-oxo-2-pyrazinecarboxamide. Its chemical formula is C5H4FN3O2, and its molecular weight is approximately 157.10 g/mol.
Favipiravir selectively inhibits the influenza viral RNA-dependent RNA polymerase.
Favipiravir Impurities and Synthesis
Impurities in Favipiravir refer to unintended substances or byproducts present in the medication. They can arise during the synthesis1, manufacturing, or storage processes. They encompass related compounds, degradation products, residual solvents, or other impurities introduced during production or handling. They can impact Favipiravir purity, stability, and overall quality. Strict regulatory guidelines and quality control measures help monitor and control the levels of impurities to ensure the safety and effectiveness of Favipiravir medications. It is essential to minimize and manage them to uphold the integrity and therapeutic value of Favipiravir.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Favipiravir impurity standards, including 3-hydroxypyrazine-2-carboxamide, 3,6-dichloropyrazine-2-carbonitrile, 6-bromo-3-hydroxypyrazine-2-carboxamide, 6-Chloro-3-hydroxypyrazine-2-carboxamide, 6-Chloroypyrazine-2-carbonitrile, Favipiravir fluoro Ribofuranose, Favipiravir Ribofuranose, and Favipiravir Ribofuranose metabolite. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Favipiravir impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Furuta, Yousuke; Egawa, Hiroyuki, Nitrogenous heterocyclic carboxamide derivatives or salt thereof as antiviral agents, Toyama Chemical Co., Ltd., Japan, EP1112743B1, October 24, 2007 (https://patents.google.com/patent/EP1112743B1/en)
- Bulduk, Ibrahim, HPLC-UV method for quantification of favipiravir in pharmaceutical formulations, Acta Chromatographica, Volume: 33, Issue: 3, Pages: 209-215, 2021
Frequently Asked Questions
How are Favipiravir impurities in drug formulations characterized?
Various physicochemical characterization techniques, such as melting point determination, solubility studies, and spectroscopic analysis, help characterize impurities in Favipiravir formulations.
Can Favipiravir impurities in drug formulations result in changes to the pharmacodynamic effects of the drug?
Some impurities in Favipiravir formulations may alter the drug's pharmacodynamic effects, affecting its mechanism of action or therapeutic outcomes.
How are Favipiravir impurities controlled during storage and transportation?
Proper storage conditions, such as temperature and humidity control, and suitable packaging materials help minimize impurity formation or degradation in Favipiravir during storage and transportation.
How should Favipiravir impurities be stored in terms of temperature?
Favipiravir impurities are stored at a controlled room temperature, 2-8 °C, or according to the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.