Etomidate
General Information
Etomidate Impurities and Etomidate
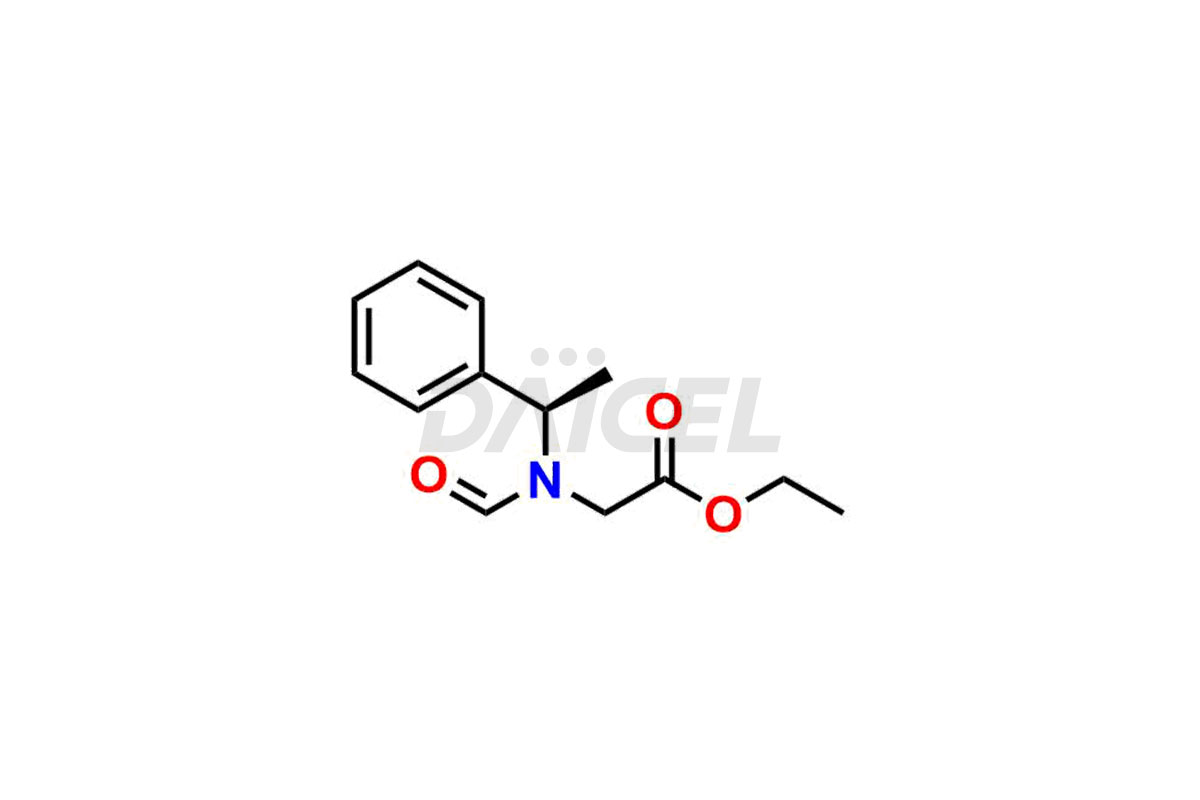
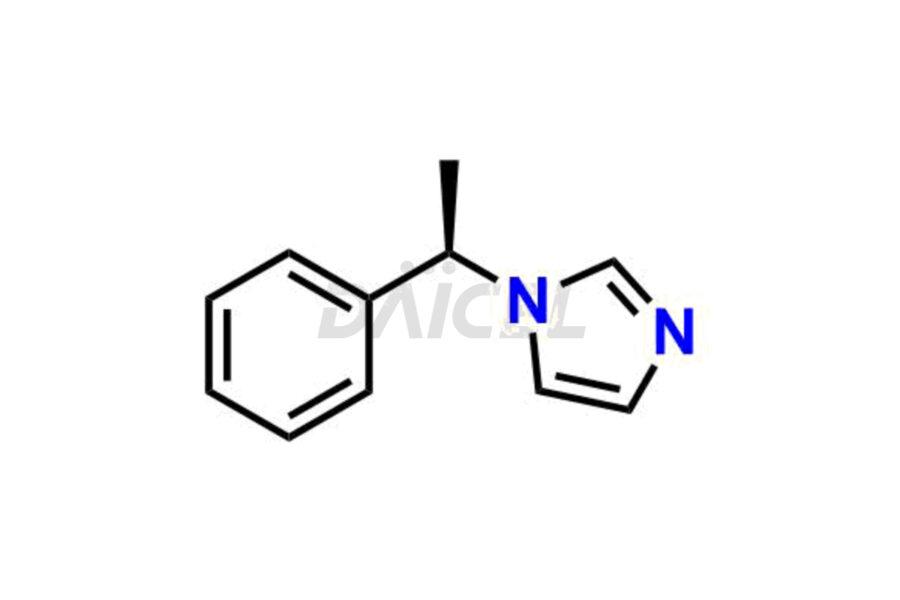
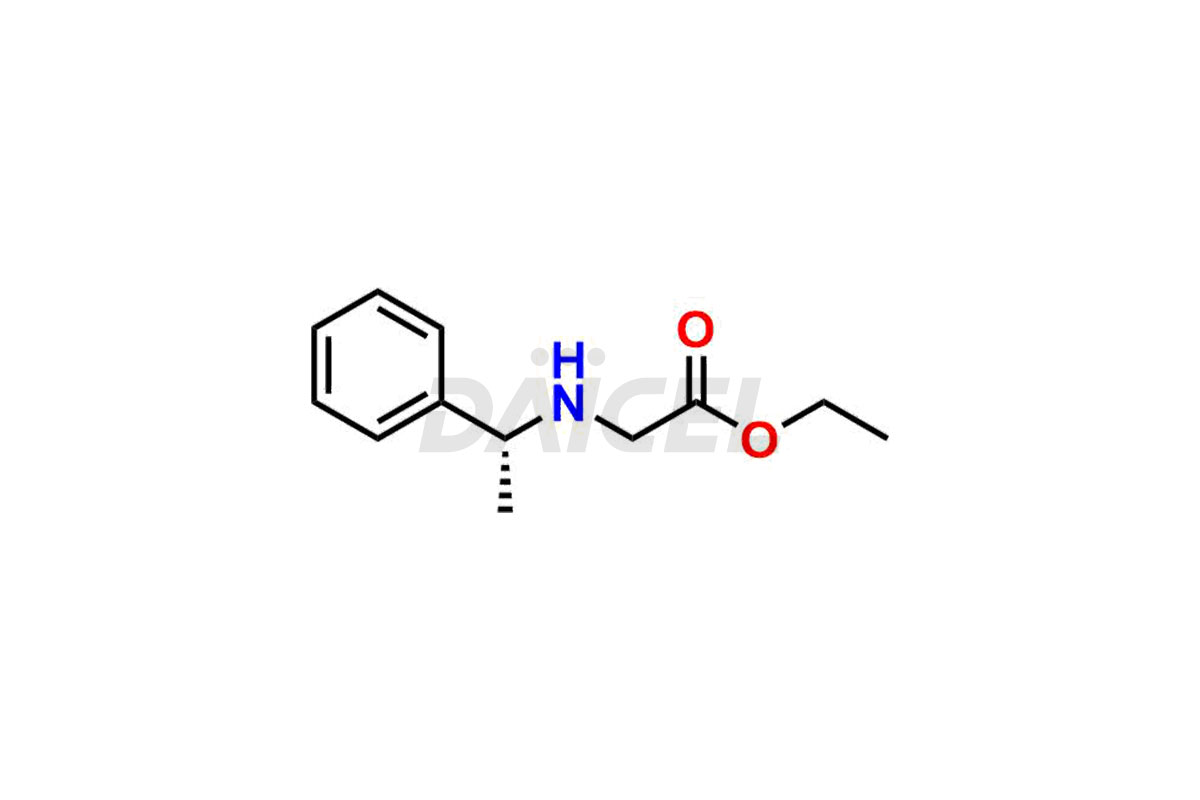
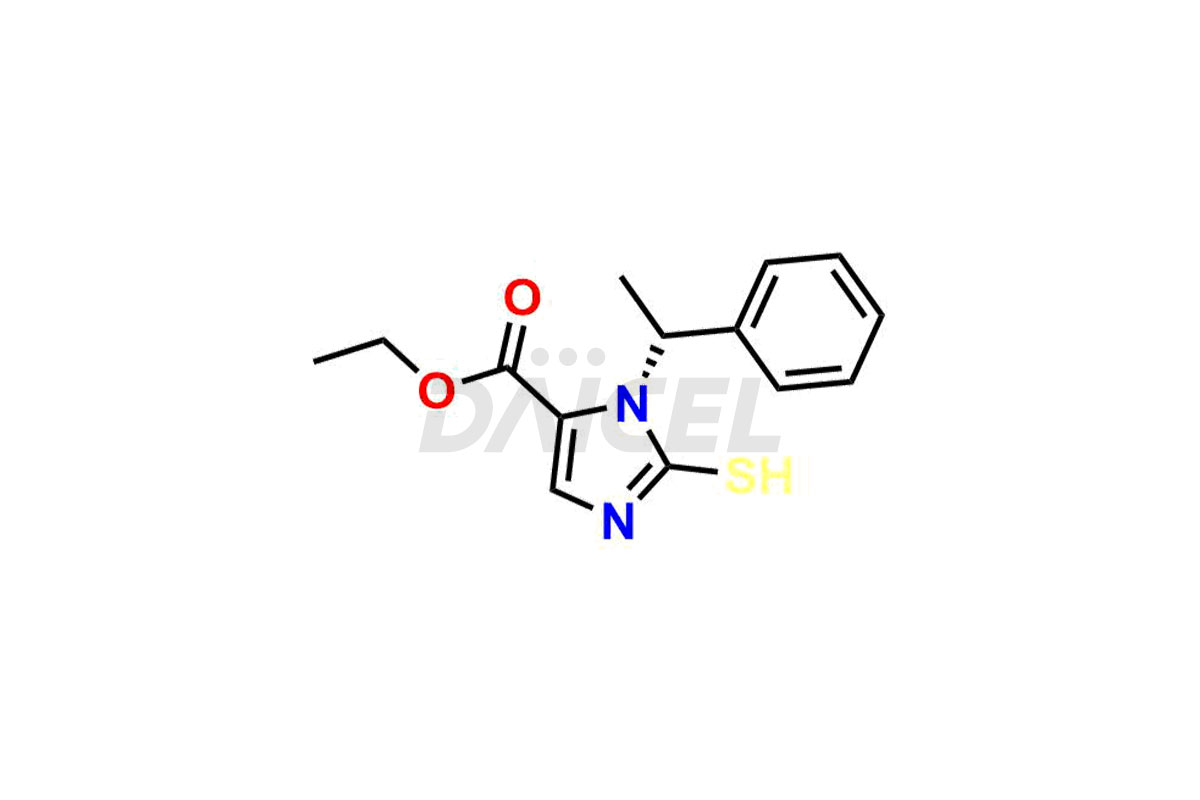
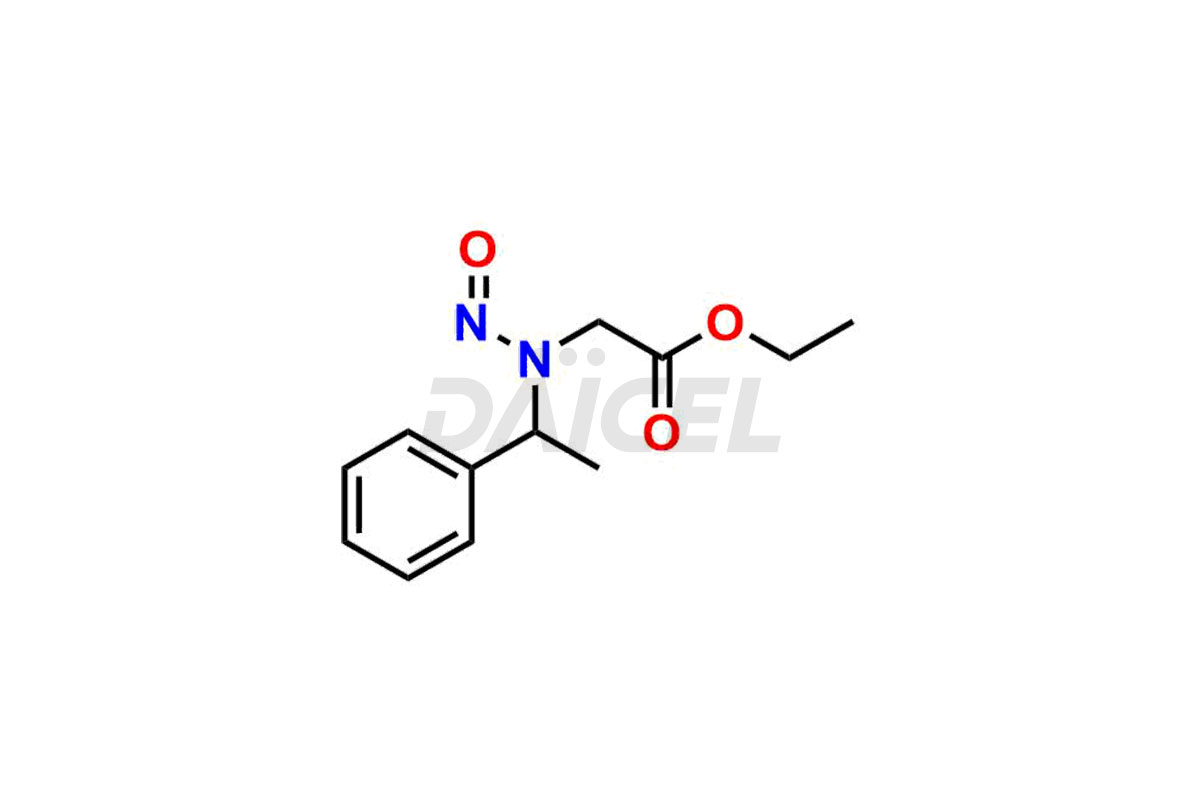
Daicel Pharma synthesizes high-quality Etomidate impurities, (R)-ethyl N-formyl-N-(1-phenylethyl) glycine, 1H-imidazole, 1-[(1R)-1-phenylethyl]-, Ethyl (R)-[(1-Phenylethyl)amino] acetate, Ethyl N-formyl-3-oxo-N-(1-phenylethyl)alaninate, Etomidate intermediate Nitroso impurity, and Ethyl (R)-3-(1-phenylethyl)-2-thioxo-23-dihydro-1H-imidazole-4-carboxylate., crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Etomidate. Moreover, Daicel Pharma offers custom synthesis of Etomidate impurities and delivers them globally.
Etomidate [CAS: 33125-97-2] is a short-acting anesthetic. It is an imidazole carboxylate derivative.
Etomidate: Use and Commercial Availability
Etomidate is an anesthetic agent that acts quickly and has no analgesic properties. It is available under the trade name AMIDATE (intravenous administration). It is an ultrashort-acting, non-barbiturate hypnotic drug used during general anesthesia, rapid sequence intubation, and procedural sedation. Etomidate is also used to maintain anesthesia and short surgical procedures, such as reducing dislocated joints, cardioversion, and tracheal intubation.
Etomidate Structure and Mechanism of Action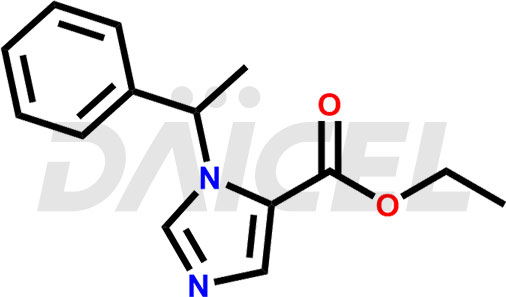
The chemical name of Etomidate is (R)-Ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate. Its chemical formula is C14H16N2O2, and its molecular weight is approximately 244.29 g/mol.
Etomidate is structurally different from other anesthetic agents. It interacts with gamma-Aminobutyric acid type A (GABAA) receptors, potentiating the effects of GABA.
Etomidate Impurities and Synthesis
Impurities in the drug substance and finished drug product may impact its safety and efficacy. So, the synthesis of Etomidate impurities helps identify, quantify, and evaluate the chemical properties and stability of the drug. Further, developing analytical methods to detect and quantify impurities is critical for drug quality control and regulatory compliance.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Etomidate impurity standards, (R)-ethyl N-formyl-N-(1-phenylethyl) glycine, 1H-imidazole, 1-[(1R)-1-phenylethyl]-, Ethyl (R)-[(1-Phenylethyl)amino] acetate, Ethyl N-formyl-3-oxo-N-(1-phenylethyl)alaninate, Etomidate intermediate Nitroso impurity, and Ethyl (R)-3-(1-phenylethyl)-2-thioxo-23-dihydro-1H-imidazole-4-carboxylate. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Etomidate impurity or degradation product.
References
FAQ's
References
- Ellis, Edwin Owen; Beck, Peter Richard, Determination of etomidate in human plasma by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 232, Issue: 1, Pages: 207-11, 1982
- Shaw, J.; Kay, B.; Keegan, M.; Healy, T. E. J., Quantitative estimation of etomidate in water, plasma and blood. A comparison between high-performance liquid chromatography and gas chromatography, Journal of Pharmacological Methods, Volume: 7, Issue: 4, Pages: 311-19, 1982
Frequently Asked Questions
What are the potential risks associated with Etomidate impurities?
The potential risks associated with Etomidate impurities are toxicity and reduced efficacy of the drug causing harmful effects on patient's health.
How are Etomidate impurities detected and quantified?
Etomidate impurities can be detected and quantified using analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and liquid chromatography-mass spectrometry (LC-MS).
Which solvent is used for the analysis of Etomidate impurities?
Methanol is commonly used for the analysis of Etomidate and its impurities. However, Acetonitrile is for tanalyzing impurities, such as Etomidate intermediate Nitroso impurity.
What are the temperature conditions required to store Etomidate impurities?
Etomidate impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.