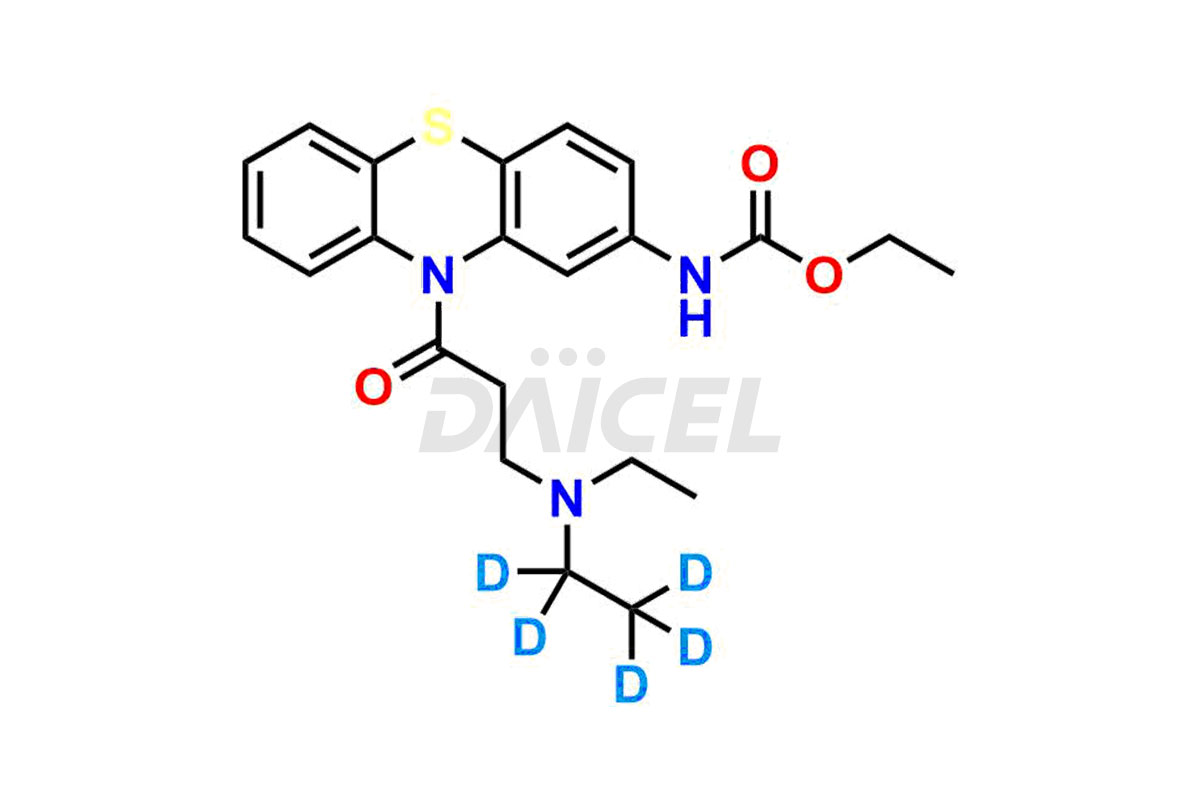
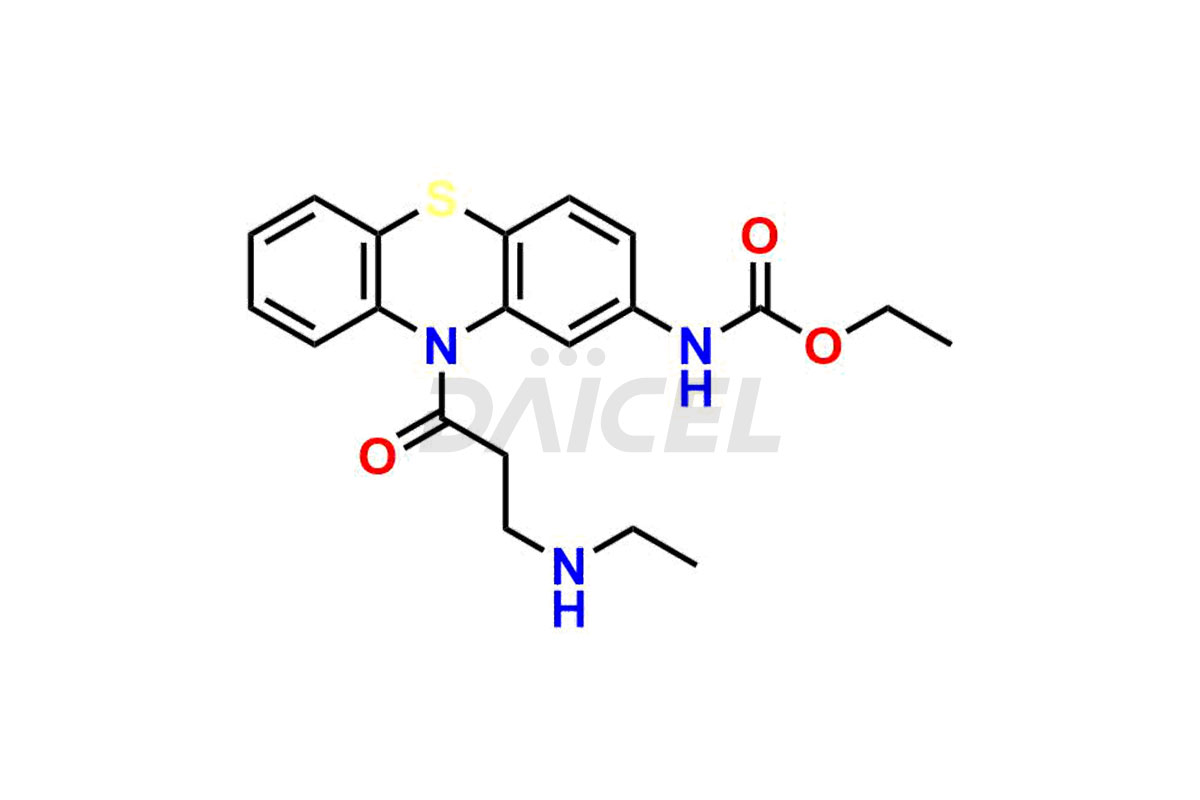
Ethacizine
General Information
Ethacizine Impurities and Ethacizine
Daicel Pharma offers high-quality impurities for Ethacizine, an active pharmaceutical ingredient. The impurity, Mono-Desethyl Ethacizine, plays a vital role in assessing the purity, reliability, and safety of Ethacizine. Daicel Pharma also offers a customized synthesis of Ethacizine impurities to cater to client requirements, with worldwide delivery options available.
Ethacizine [CAS: 33414-33-4], a related compound to moracizine, belongs to the class Ic antiarrhythmic agents. Its primary use is in Russia and other Commonwealth of Independent States (CIS) countries. It manages ventricular and supraventricular arrhythmias that are severe or refractory, particularly when accompanied by underlying heart disease. Additionally, it treats refractory tachycardia associated with Wolff-Parkinson-White syndrome.
Ethacizine: Use and Commercial Availability
Ethacizine, a Class Ic antiarrhythmic drug, treats recurrent sustained ventricular tachycardia (VT). Ethacizine is effective for treating VT in coronary artery disease patients and is vital in programmed electrical stimulation in VT management. However, more research is needed to determine Ethacizine’s effectiveness in other heart diseases.
Ethacizine Structure and Mechanism of Action 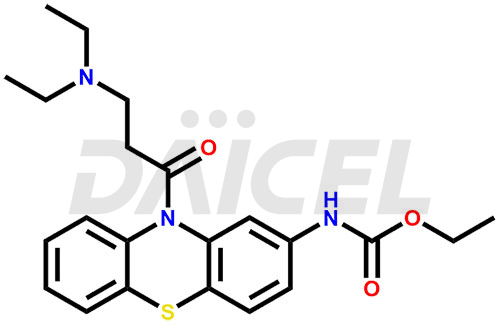
The chemical name of Ethacizine is Ethyl N-[10-[3-(diethylamino)-1-oxopropyl]-10H-phenothiazin-2-yl] carbamate. Its chemical formula is C22H27N3O3S, and its molecular weight is approximately 413.5 g/mol.
Ethacizine suppresses retrograde stimulus conduction along the atrioventricular node and helps prevent paroxysmal atrioventricular nodal tachycardia.
Ethacizine Impurities and Synthesis
Ethacizine impurities refer to the presence of unwanted substances or byproducts found in Ethacizine drugs. They arise during the synthesis1 or manufacturing process of Ethacizine. The common Ethacizine impurities may include related compounds, residual solvents, degradation products, and those introduced during synthesis or formulation. They can affect the purity, stability, and overall quality of Ethacizine. Therefore, it is vital to closely monitor and control the levels of impurities to ensure the safety and efficacy of Ethacizine medications.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for the Ethacizine impurity standard, Mono-Desethyl Ethacizine. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Ethacizine impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Ethacizine. Ethacizine-D5, a deuterium-labeled Ethacizine standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
References
FAQ's
Frequently Asked Questions
How are Ethacizine impurities analyzed?
Various analytical techniques, such as chromatography and spectroscopy, help detect and quantify impurities in Ethacizine.
What are the measures to prevent impurity formation in Ethacizine?
Good manufacturing practices, proper raw material handling, and adequate purification steps prevent impurity formation in Ethacizine.
Which solvent helps in analyzing Ethacizine impurities?
Methanol is the solvent used for analyzing most of the impurities in Ethacizine.
How should Ethacizine impurities be stored in terms of temperature?
Ethacizine impurities are stored at a controlled room temperature, 2-8 °C, or according to the specifications provided on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.