LOAD MORE
You're viewed 9 of 18 products
Daicel Pharma synthesizes high-quality Erlotinib impurities, Desmethyl Erlotinib acetate, Erlotinib acetate, Erlotinib EP impurity I, Erlotinib N-Oxide, Erlotinib Related Compound A, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Erlotinib. Moreover, Daicel Pharma offers custom synthesis of Erlotinib impurities and delivers them globally.
Erlotinib [CAS: 183321-74-6] is a quinazolinamine derivative. It is a Human Epidermal Growth Factor Receptor Type 1/Epidermal Growth Factor Receptor (HER1/EGFR) tyrosine kinase inhibitor. It is for treating advanced or metastatic forms of pancreatic or non-small cell lung cancer.
Erlotinib, sold under the trade name TARCEVA, is a medicine used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. For NSCLC, Erlotinib is recommended as a first, second, or third-line therapy based on the patient’s characteristics. Erlotinib is a tyrosine kinase receptor inhibitor that is particularly effective against cancers with EGFR mutations.
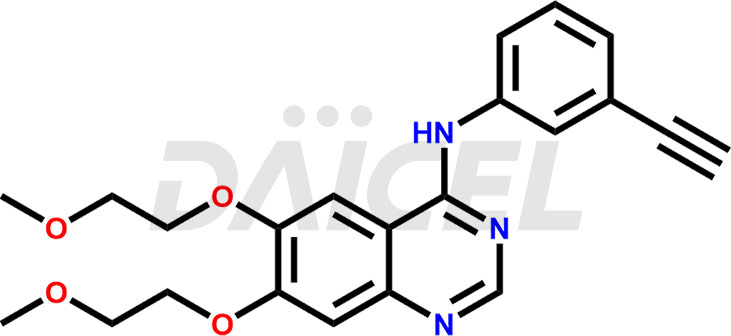
The chemical name of Erlotinib is N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. Its chemical formula is C22H23N3O4, and its molecular weight is approximately 393.4 g/mol.
Erlotinib inhibits the intracellular phosphorylation of tyrosine kinase linked with the epidermal growth factor receptor (EGFR). However, the mechanism of the anti-tumor action of Erlotinib is not completely characterized.
During the synthesis1 of Erlotinib, impurities may form, which can affect the quality and safety of the drug. The identification and detection of these impurities are vital to ensure the effectiveness and safety of Erlotinib. The impurities form due to starting materials, synthetic intermediates, reactions, or storage conditions.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Erlotinib impurity standards, Desmethyl Erlotinib acetate, Erlotinib acetate, Erlotinib EP impurity I, Erlotinib N-Oxide, Erlotinib Related Compound A, etc. The CoA includes characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Erlotinib impurity or degradation product. We also provide labeled compounds to quantify the efficacy of Erlotinib. Daicel offers Erlotinib-D6, a deuterium-labeled Erlotinib standard used in bio-analytical research, such as BA/BE studies with isotope data in CoA.
The rapid, reversed-phase high-performance liquid chromatographic (HPLC) method helps detect and analyze Erlotinib and its impurities.
Erlotinib is stored in a cool, dry place and protected from light and moisture to prevent the formation of impurities.
Methanol is one of the solvents used in analyzing most of the Erlotinib impurities.
Erlotinib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.