LOAD MORE
You're viewed 9 of 18 products
Daicel Pharma synthesizes more than fifteen high-quality Enzalutamide impurities, such as C-Desmethyl Enzalutamide acid impurity, Des Fluoro Enzalutamide, ENZ cyano desfluoro impurity, ENZ DIAMIDE Impurity, Enzalutamide Carboxylic Acid, N-Desmethyl Enzalutamide, Oxo-Enzalutamide and more, crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Enzalutamide. Moreover, Daicel Pharma offers custom synthesis of Enzalutamide impurities and delivers them globally.
Enzalutamide [CAS: 915087-33-1] is a nonsteroidal androgen receptor inhibitor treatingmetastatic castration-resistant prostate cancer. Enzalutamide acts as an antineoplasticagent and inhibits androgen activity.
Enzalutamide is a US FDA-approved medicine that treats patients with metastatic castration-resistant prostate cancer where hormone therapy has failed. Enzalutamide is sold under the brand name Xtandi.
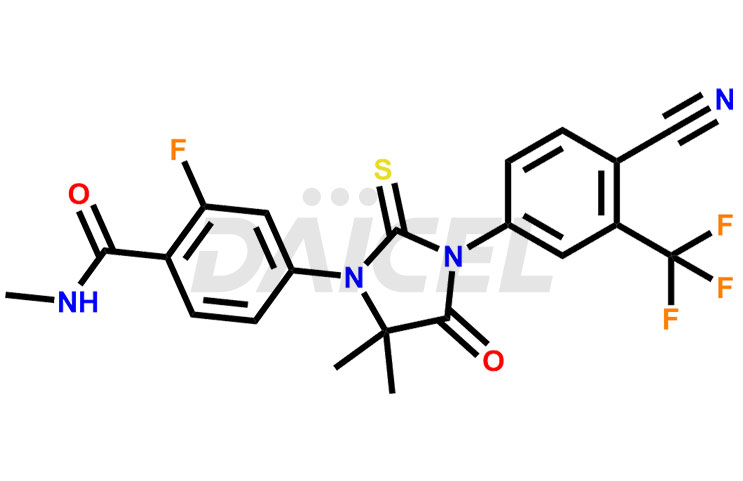
The chemical name of Enzalutamide is 4-[3-[4-Cyano-3-(trifluoromethyl) phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methylbenzamide. Its chemical formula is C21H16F4N4O2S, and its molecular weight is approximately 464.4g/mol.
Enzalutamide is an androgen receptor (AR) inhibitor, classified as a second-generation drug, and acts on various steps in the androgen receptor signaling pathway. It inhibits androgen binding to androgen receptors. In addition, it inhibits androgen receptor nuclear translocation and DNA interaction.
During its synthesis1 or storage, impurities form in Enzalutamide, which can affect the drug’s efficacy and safety. Related substances, starting materials, intermediates, and degradation products are present as impurities in Enzalutamide. Impurity formation is due to impurities in the starting materials, poor reaction conditions, or exposure to light and heat during storage. Thus, it is essential to monitor and control Enzalutamide impurities to ensure the quality and safety of the drug.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Enzalutamide impurity standards, including C-Desmethyl Enzalutamide acid impurity, Des Fluoro Enzalutamide, ENZ cyano desfluoro impurity, ENZ DIAMIDE Impurity, Enzalutamide Carboxylic Acid, N-Desmethyl Enzalutamide, Oxo- Enzalutamide, etc. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We also provide 13C-DEPT and CHN when requested. We also give a complete characterization report. Daicel has the technology and expertise to prepare any unknown Enzalutamide impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Enzalutamide. Daicel offers Enzalutamide D3 and Enzalutamide D6, which are deuterated-labeled standards of Enzalutamide for bioanalytical research and BA/BE studies with isotope data in CoA.
Enzalutamide impurities are monitored regularly during drug development, manufacturing, and post-marketing surveillance to ensure the quality and safety of the drug.
Enzalutamide impurities affect patient safety due to toxicity, reduced efficacy, and increased risk of side effects.
Purification techniques, such as recrystallization and chromatography, help reduce the level of impurities in Enzalutamide.
N-desmethyl Enzalutamide, a known metabolite of the drug, is an impurity of concern. It has been found to have anti-androgenic activity and could potentially reduce the efficacy of Enzalutamide.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.