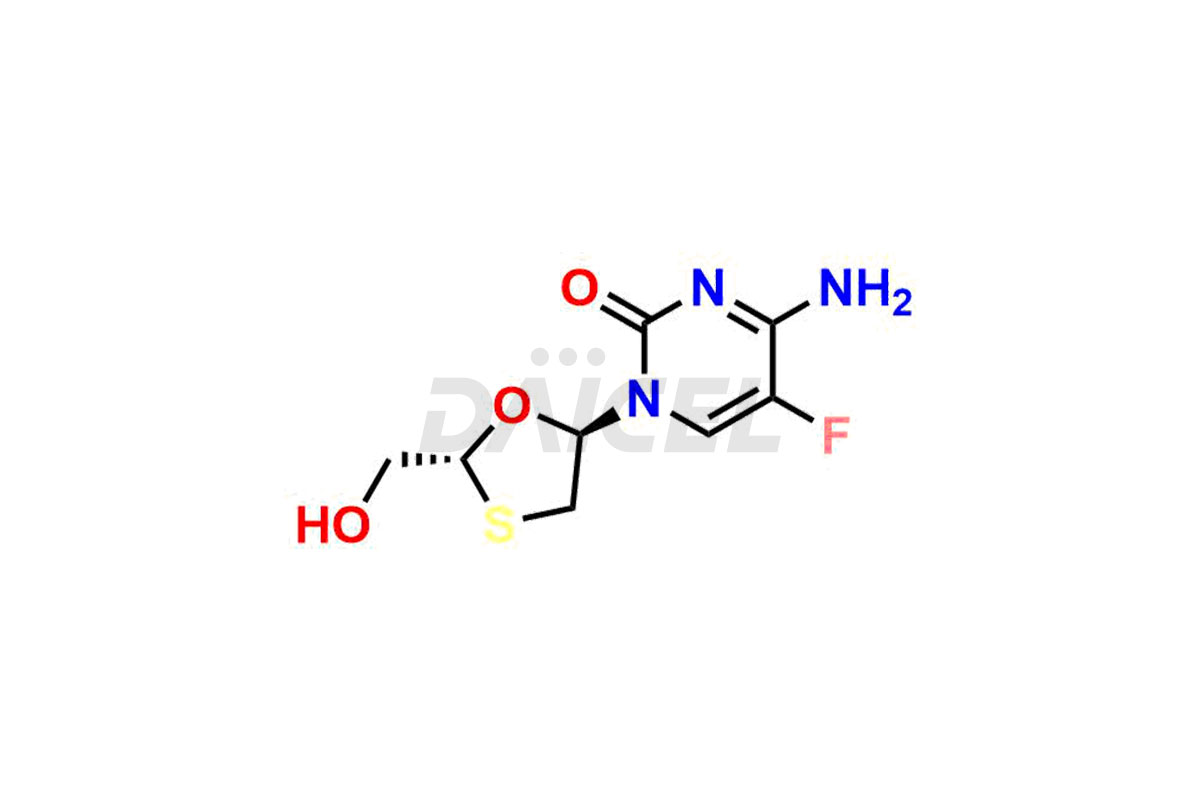
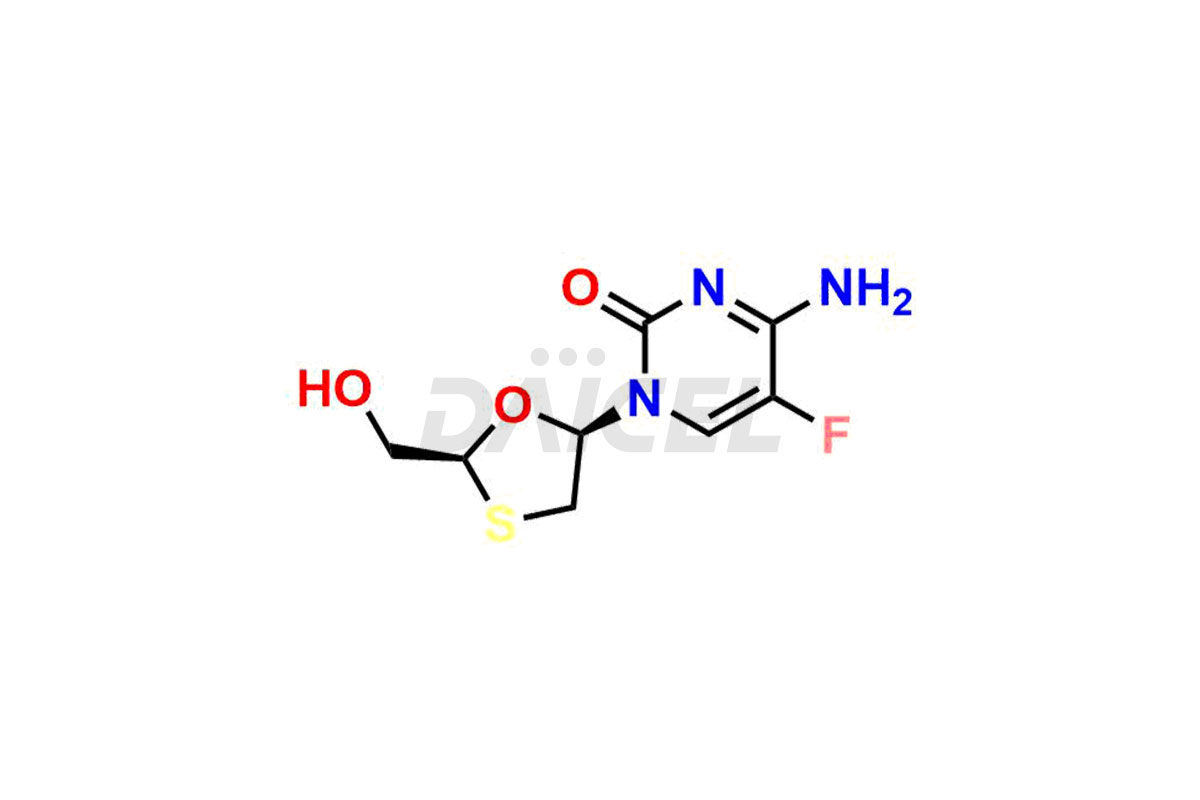
Emtricitabine
General Information
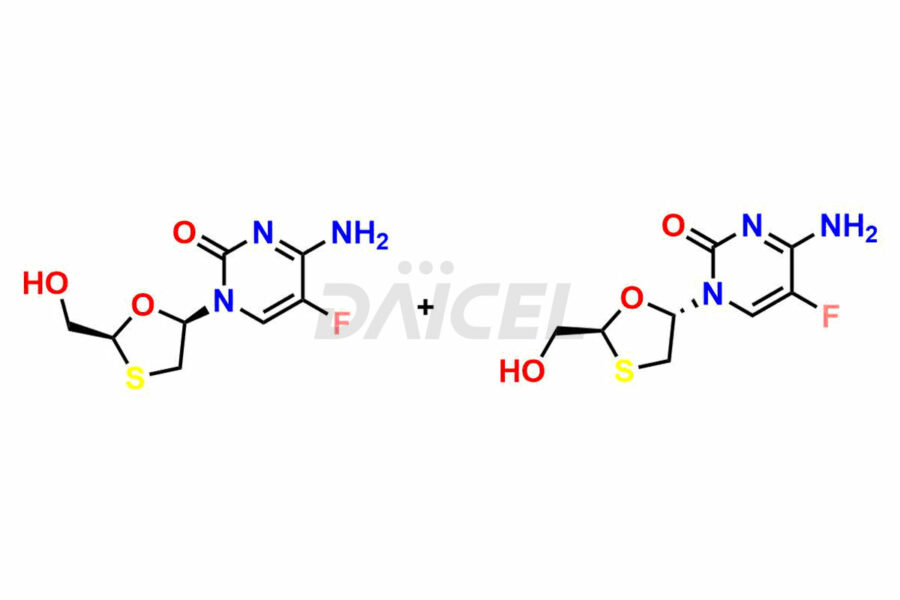
Emtricitabine Impurities and Emtricitabine
Daicel Pharma synthesizes high-quality Emtricitabine impurities, (2R,5R)-Emtricitabine, (2S,5R)-Emtricitabine, (2S,5S)-Emtricitabine and Emtricitabine Diastereomer, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Emtricitabine. Moreover, Daicel Pharma offers custom synthesis of Emtricitabine impurities and delivers them globally.
Emtricitabine [CAS: 143491-57-0] is a synthetic nucleoside analog possessing antiviral properties. It is the (-) enantiomer of a thio analog of cytidine.
Emtricitabine: Use and Commercial Availability
Emtricitabine is a nucleoside reverse transcriptase inhibitor. It is effective against both human immunodeficiency virus (HIV) and hepatitis B virus. , In combination with other antiretroviral drugs, Emtricitabine treats HIV infection in adults. It effectively reduces or maintains viral load suppression in antiretroviral naïve adults or experienced patients switching from stable combination regimes. Emtricitabine is a preferred choice for patients coinfected with HIV and hepatitis B virus. It is an active ingredient in drugs such as Emtriva, Atripla, Biktarvy, Complera, Descovy Genvoya, Odefsey, Stribild, Symtuza, Truvada, and others.
Emtricitabine Structure and Mechanism of Action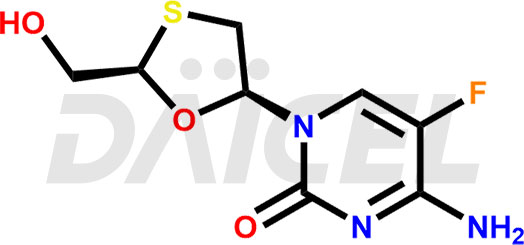
The chemical name of Emtricitabine is 4-Amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone. Its chemical formula is C8H10FN3O3S, and its molecular weight is approximately 247.25 g/mol.
Emtricitabine is phosphorylated by cellular enzymes giving emtricitabine 5’-triphosphate. It inhibits the activity of HIV-1 reverse transcriptase and incorporates it into nascent viral DNA resulting in chain termination.
Emtricitabine Impurities and Synthesis
During the synthesis1 and subsequent storage of Emtricitabine, impurities can form through various mechanisms such as oxidation, deamination, and isomerization. These impurities may affect the stability and efficacy of Emtricitabine, and their presence must be monitored and controlled during manufacturing.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Emtricitabine impurity standards, (2R,5R)-Emtricitabine, (2S,5R)-Emtricitabine, (2S,5S)-Emtricitabine, and Emtricitabine Diastereomer. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Emtricitabine impurity or degradation product.
References
FAQ's
References
- Liotta, Dennis C.; Schinazi, Raymond F.; Choi, Woo Baeg, Method for the synthesis, compositions and use of 2'-deoxy-5-fluoro-3'-thiacytidine and related compounds, Emory University, United States, US5814639A, September 29, 1998
- Shockcor, J. P.; Wurm, R. M.; Frick, L. W.; Sanderson, P. N.; Farrant, R. D.; Sweatman, B. C.; Lindon, J. C., HPLC-NMR identification of the human urinary metabolites of (-)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine, a nucleoside analog active against human immunodeficiency virus (HIV), Xenobiotica, Volume: 26, Issue: 2, Pages: 189-99, 1996
Frequently Asked Questions
How are degradation impurities formed in Emtricitabine?
Under acidic, alkaline, and oxidative conditions, Emtricitabine is susceptible to degradation, with higher susceptibility observed under oxidative conditions. This degradation leads to the formation of various impurities, which may affect the quality and efficacy of the drug.
How can Emtricitabine impurities be detected?
Analytical methods such as high-performance liquid chromatography, liquid chromatography (LC) method coupled with ultraviolet (UV) detection, and LC-MS help detect and characterize Emtricitabine impurities.
Which solvent is for the analysis of Emtricitabine impurities?
Methanol is a solvent for the analysis of Celecoxib impurities.
What are the temperature conditions required to store Emtricitabine impurities?
Emtricitabine impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.