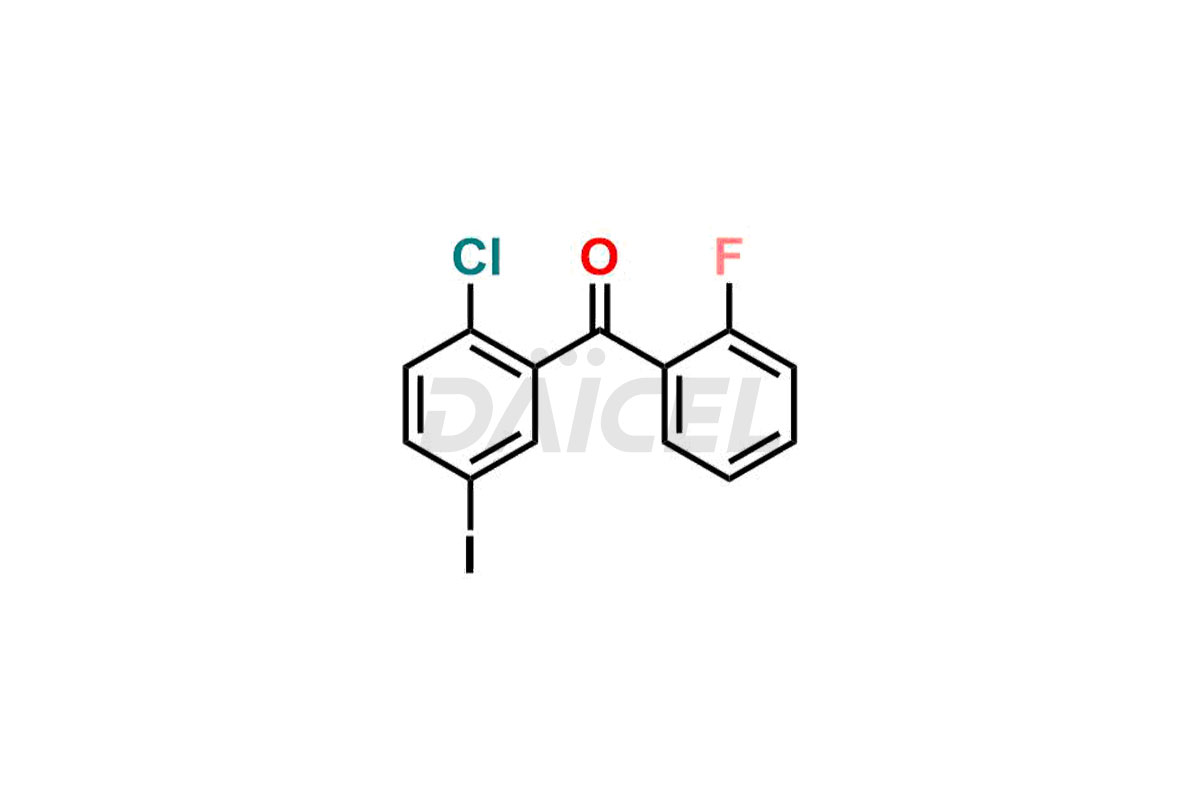
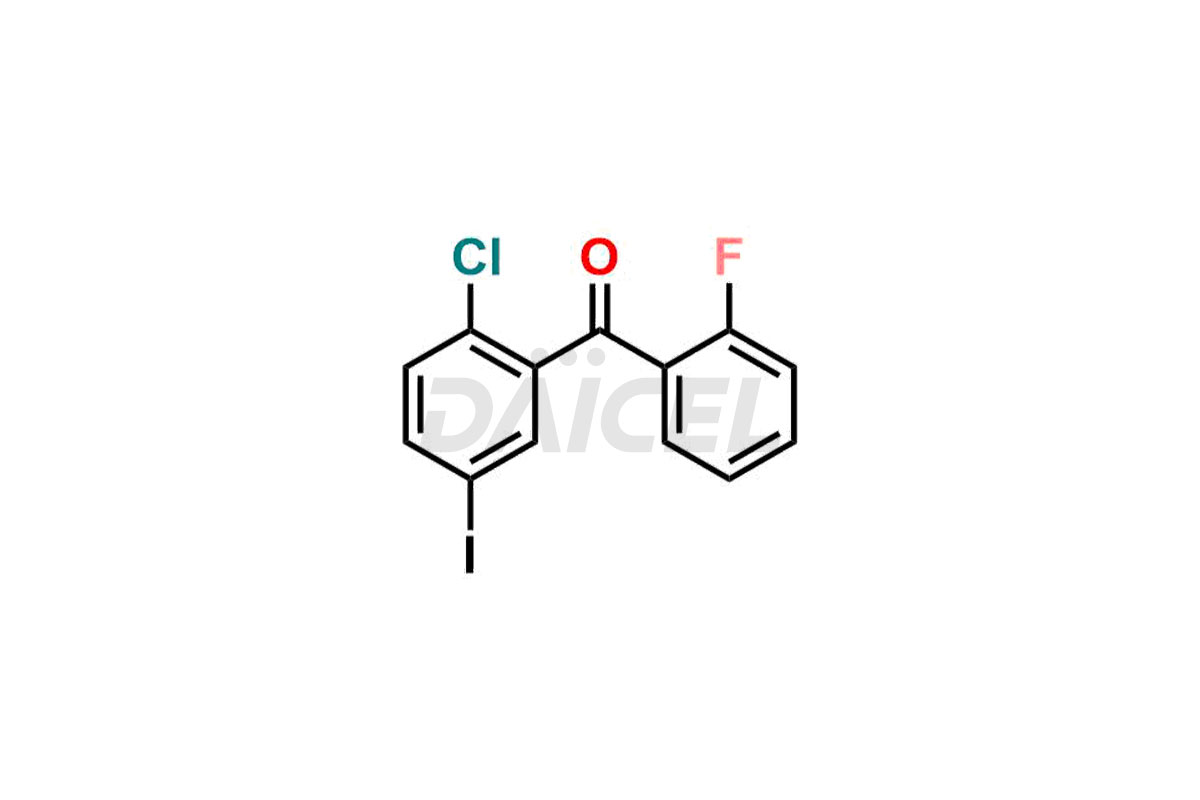
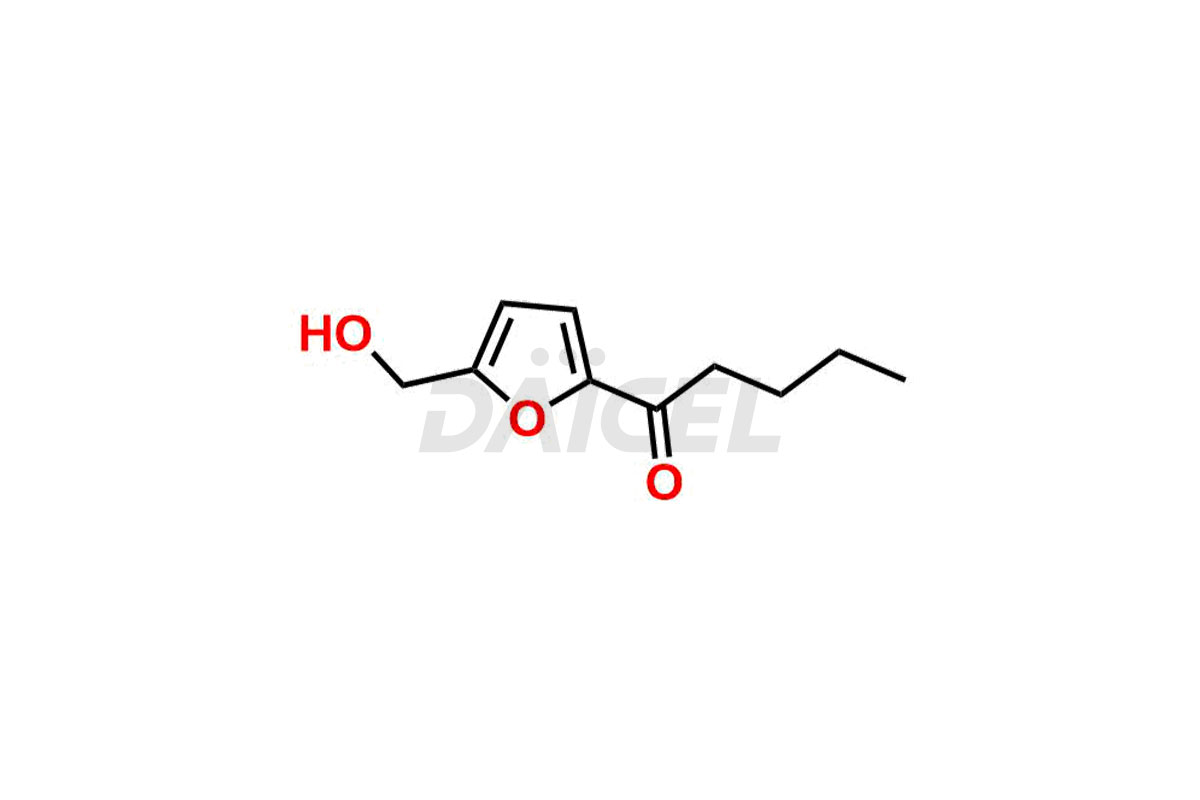
Empagliflozin
General Information
Empagliflozin Impurities and Empagliflozin
Daicel Pharma offers high-quality impurities for Empagliflozin, an active pharmaceutical ingredient. These impurities, including (2-chloro-5-iodophenyl)(2-fluoro-4-methylphenyl)methanone, (2-chloro-5-iodophenyl)(2-fluoro-5-methylphenyl)methanone, (2-chloro-5-iodophenyl)(2-fluorophenyl)methanone, (2,5-diiodophenyl)(2-fluorophenyl)methanone, and Empagliflozin Impurity 9, play a vital role in assessing the purity, reliability, and safety of Empagliflozin. Daicel Pharma also offers a customized synthesis of Empagliflozin impurities to cater to client requirements, with worldwide delivery options available.
Empagliflozin [CAS: 864070-44-0] is a medication that works by competitively inhibiting sodium-glucose co-transporter 2 (SGLT2; SLC5A2), which is primarily responsible for the reabsorption of glucose in the kidney It acts as a hypoglycemic agent and is a sodium-glucose transport protein subtype 2 inhibitors. It is also used in combination with other drug therapies. It is an adjunct to diet and exercise for treating type 2 diabetes mellitus.
Empagliflozin: Use and Commercial Availability
Empagliflozin, marketed under Jardiance, is an antidiabetic agent for treating type 2 diabetes mellitus in adults. It is used alone or with other antidiabetic medications, including linagliptin and metformin. In 2016, the FDA approved an additional indication for Empagliflozin to reduce the risk of cardiovascular death in adult patients with type 2 diabetes and cardiovascular disease. Empagliflozin has demonstrated efficacy in reducing hospitalizations for heart failure and cardiovascular mortality.
Empagliflozin Structure and Mechanism of Action 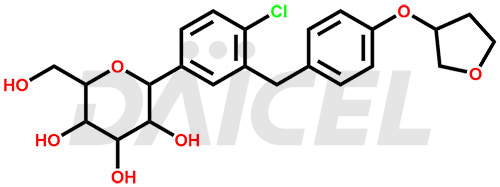
The chemical name of Empagliflozin is (1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol. Its chemical formula is C23H27ClO7, and its molecular weight is approximately 450.9 g/mol.
Empagliflozin inhibits Sodium-glucose co-transporter 2 (SGLT2) responsible for glucose reabsorption from the glomerular filtrate back into the circulation. It increases urinary glucose excretion.
Empagliflozin Impurities and Synthesis
Empagliflozin impurities are byproducts or substances present with the drug compound, Empagliflozin1. The synthesis, analysis, and control of these impurities are crucial to ensure the safety and efficacy of the medication. The synthesis of Empagliflozin impurities involves identifying the potential pathways and reactions that can lead to their formation. Analytical methods are employed to detect and quantify these impurities in Empagliflozin, using techniques like high-performance liquid chromatography (HPLC) and mass spectrometry. Strict control measures are implemented during the manufacturing process to minimize impurities and maintain the quality of Empagliflozin.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Empagliflozin impurity standards, including (2-chloro-5-iodophenyl)(2-fluoro-4-methylphenyl)methanone, (2-chloro-5-iodophenyl) (2-fluoro-5-methylphenyl)methanone, (2-chloro-5-iodophenyl)(2-fluorophenyl)methanone, (2,5-diiodophenyl)(2-fluorophenyl)methanone, and Empagliflozin Impurity 9. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Empagliflozin impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Eckhardt, Matthias; Eickelmann, Peter; Himmelsbach, Frank; Barsoumian, Edward Leon; Thomas, Leo, Glucopyranosyl-substituted phenyl derivatives, medicaments containing such compounds, their use and process for their manufacture, Boehringer Ingelheim International GmbH, Germany, US7579449B2, August 25, 2009
- Padmaja, N.; Veerabhadram, G., Development and validation of a novel stability-indicating RP-HPLC method for the determination of empagliflozin in bulk and pharmaceutical dosage form, International Journal of Pharmaceutical Sciences and Research, Volume: 7, Issue: 11, Pages: 4523-4530, 2016
Frequently Asked Questions
How are Empagliflozin impurities analyzed?
Analytical methods such as high-performance liquid chromatography (HPLC) and mass spectrometry help analyze and detect Empagliflozin impurities.
How are Empagliflozin impurities controlled during manufacturing?
Strict control measures, including process optimization, purification steps, and monitoring, are implemented during the manufacturing process to minimize impurity formation and maintain the quality of Empagliflozin.
What solvent help in analyzing Empagliflozin impurities?
Methanol or Acetonitrile are the solvents used for analyzing many impurities in Empagliflozin.
How should Empagliflozin impurities be stored in terms of temperature?
Empagliflozin impurities are stored at a controlled room temperature, between 2-8 ⁰C, or according to the specifications provided on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.