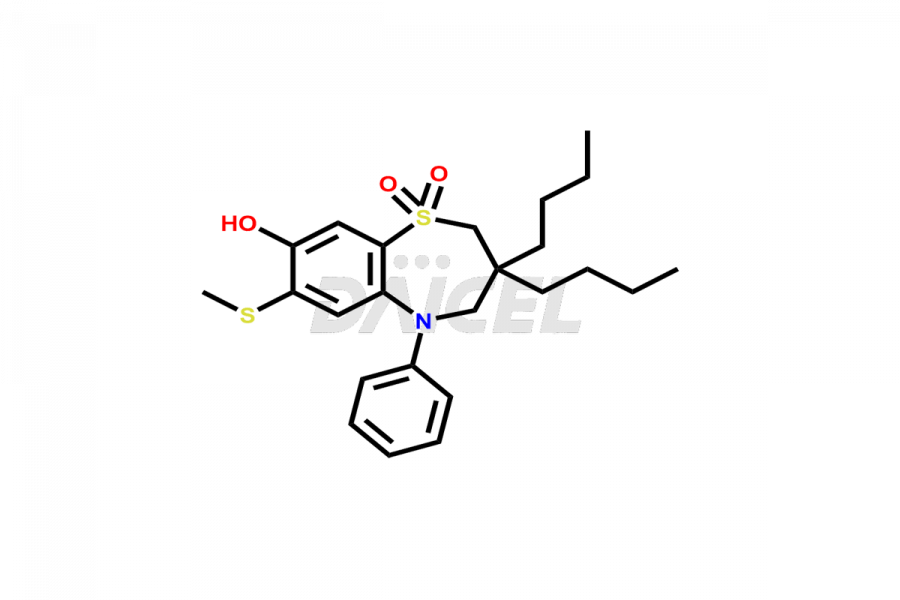
Elobixibat
General Information
Elobixibat Impurities and Elobixibat
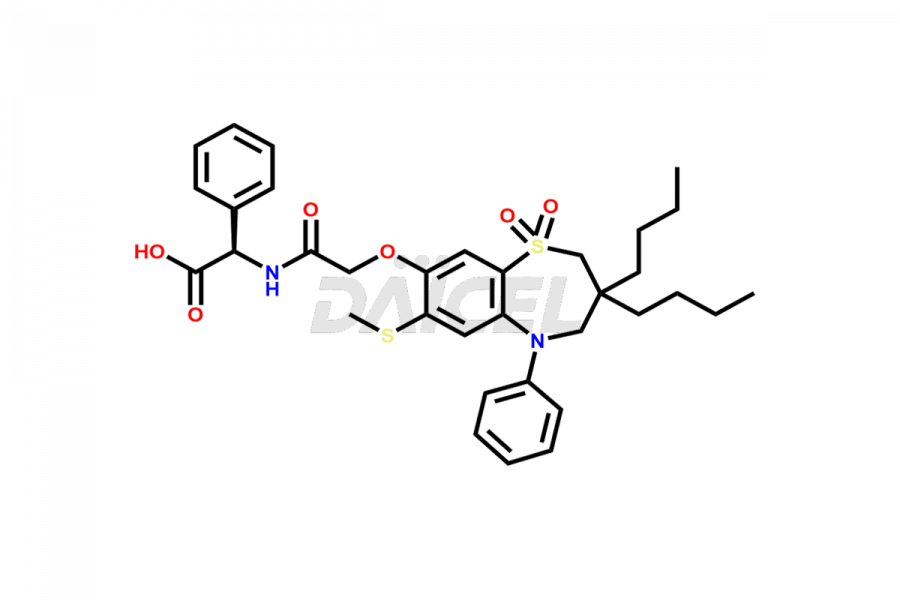
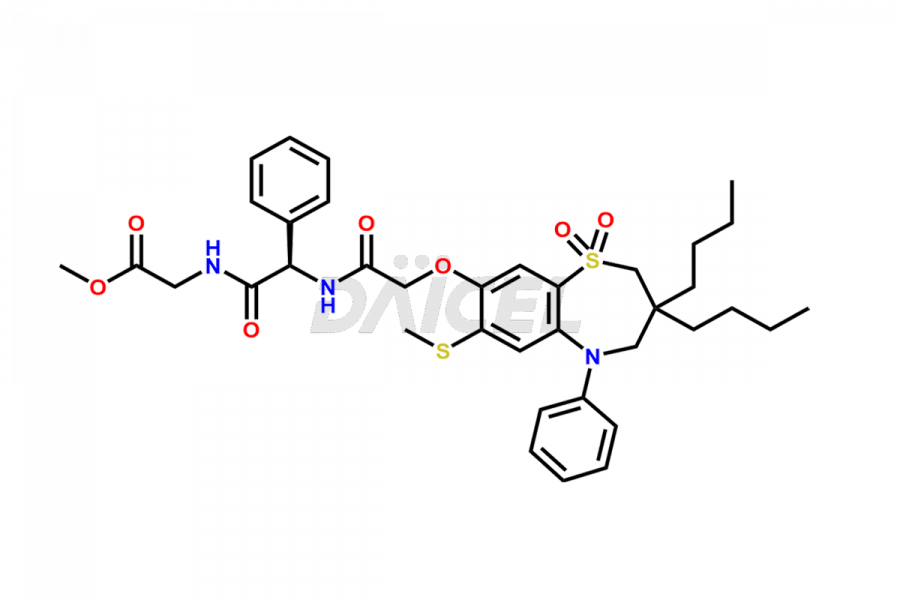
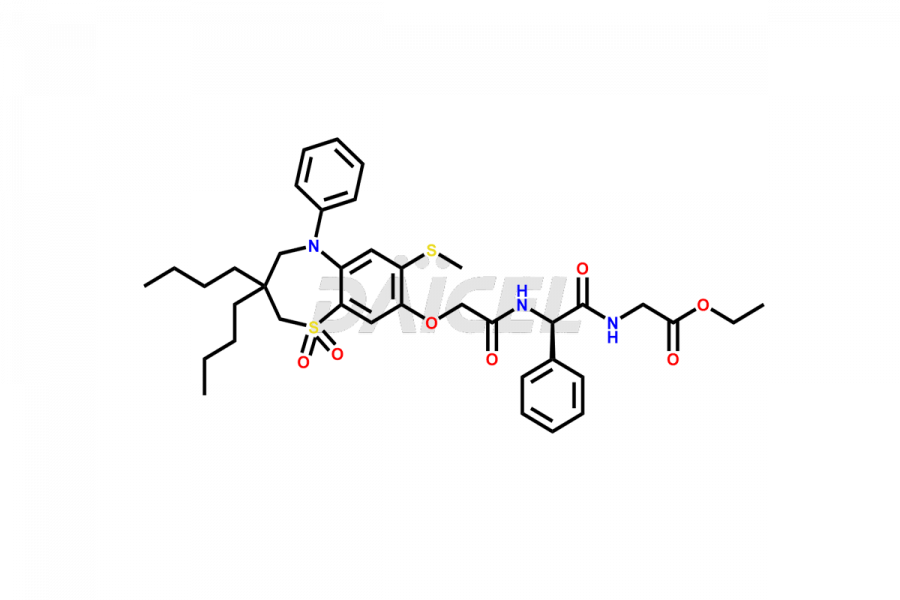
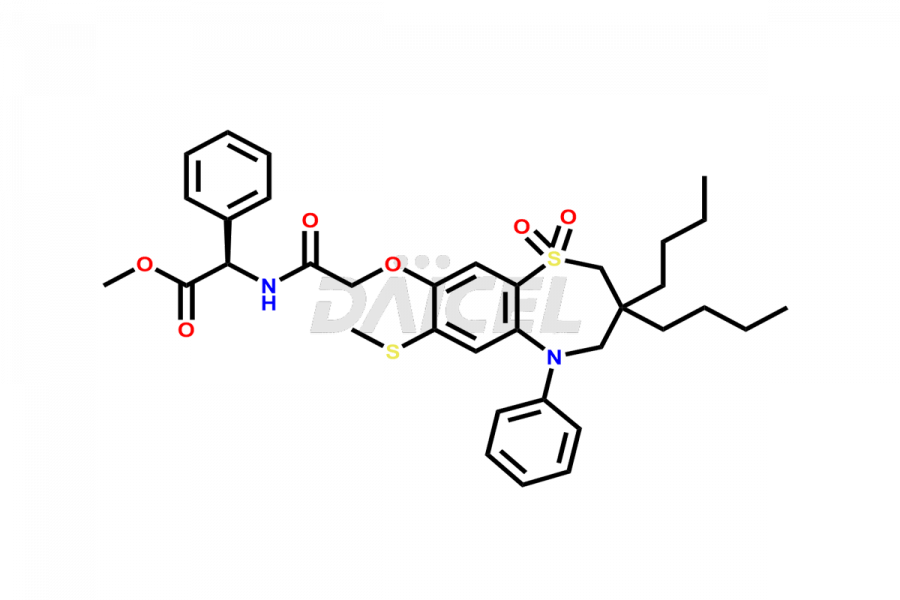
Daicel Pharma offers high-quality impurities for Elobixibat, an active pharmaceutical ingredient. These impurities, including Elobixibat acid Impurity, Elobixibat Acid Methyl Ester Impurity, Elobixibat Methyl Ester Impurity, and Elobixibat Phenol Impurity, play a vital role in assessing the purity, reliability, and safety of Elobixibat. Daicel Pharma also offers a customized synthesis of Elobixibat impurities to cater to client requirements, with worldwide delivery options available.
Elobixibat [CAS: 439087-18-0] treats Dyslipidemia, Constipation, Chronic Constipation, Functional Constipation, and Chronic Idiopathic Constipation. Elobixibat modulates the circulation of bile acids (BAs) within the enterohepatic system. It enhances the transportation of BAs to the colon, where they induce secretory and motor effects.
Elobixibat: Use and Commercial Availability
Elobixibat, available under Goofice, is a first-in-class drug for treating chronic constipation and constipation-predominant irritable bowel syndrome (IBS-C). It acts by inhibiting the ileal bile acid transporter, reducing the absorption of bile acids in the ileum. It allows bile to stimulate colonic secretion and contractions, thereby accelerating the movement of the colon. Elobixibat is minimally absorbed into the bloodstream when taken orally.
Elobixibat Structure and Mechanism of Action 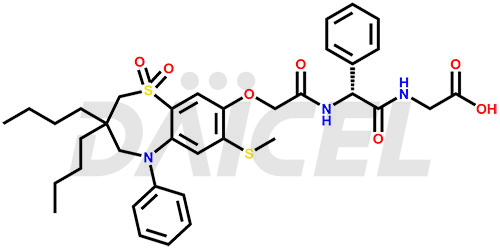
The chemical name of Elobixibat is (2R)-N-[2-[[3,3-Dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,5-benzothiazepin-8-yl]oxy]acetyl]-2-phenylglycylglycine. Its chemical formula is C36H45N3O7S2, and its molecular weight is approximately 695.9 g/mol.
Elobixibat inhibits the ileal bile acid transporter and accelerates colonic transit.
Elobixibat Impurities and Synthesis
The analysis and control of impurities in Elobixibat, a medication used for chronic constipation, is vital to ensure its safety and efficacy. Analytical techniques such as chromatography, spectroscopy, and mass spectrometry help identify and quantify impurities in Elobixibat. Impurity profiling aids in understanding the chemical composition, structure, and potential hazards associated with drug impurities. Stringent control measures during the manufacturing process1 help minimize impurity formation. Regulatory guidelines establish acceptable limits for Elobixibat impurities in pharmaceutical products, and thorough analysis and control are crucial for maintaining their quality and therapeutic effectiveness of patients.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Elobixibat impurity standards, including Elobixibat acid Impurity, Elobixibat Acid Methyl Ester Impurity, Elobixibat Methyl Ester Impurity, and Elobixibat Phenol Impurity. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Elobixibat impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Starke, Ingemar; Dahlstrom, Mikael; Blomberg, David, 1,5 Benzothiazepines and Their Use As Antihyperlipidemics, AstraZeneca AB, Sweden, EP1345918B1, April 18, 2007
- Elagamy, Samar H.; Mansour, Fotouh R.; Elbastawissy, Almoataz Bellah B.; EL-Malla, Samah F., Development of stability indicating reversed-phase high-performance liquid chromatography method for determination of Elobixibat in pure form and laboratory prepared tablets: Application to dissolution study, Journal of Separation Science, Volume: 45, Issue: 18, Pages: 3529-3541, 2022
Frequently Asked Questions
How are the Elobixibat impurities quantified?
Impurities in Elobixibat are quantified using validated analytical methods that involve calibration with appropriate reference standards and comparison of peak areas or responses. These methods help determine the levels of impurities present in the drug.
Can Elobixibat impurities be removed completely?
While efforts are ongoing to minimize impurities in Elobixibat, their complete removal is often challenging. However, the goal is to control impurity levels within acceptable limits to ensure the drug's quality and patient safety.
What solvent help in analyzing Elobixibat impurities?
Methanol is the solvent used for analyzing many impurities in Elobixibat.
How should Elobixibat impurities be stored in terms of temperature?
Elobixibat impurities are stored at a controlled room temperature, 2-8 °C, or, according to the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.