Edaravone
General Information
Edaravone Impurities and Edaravone
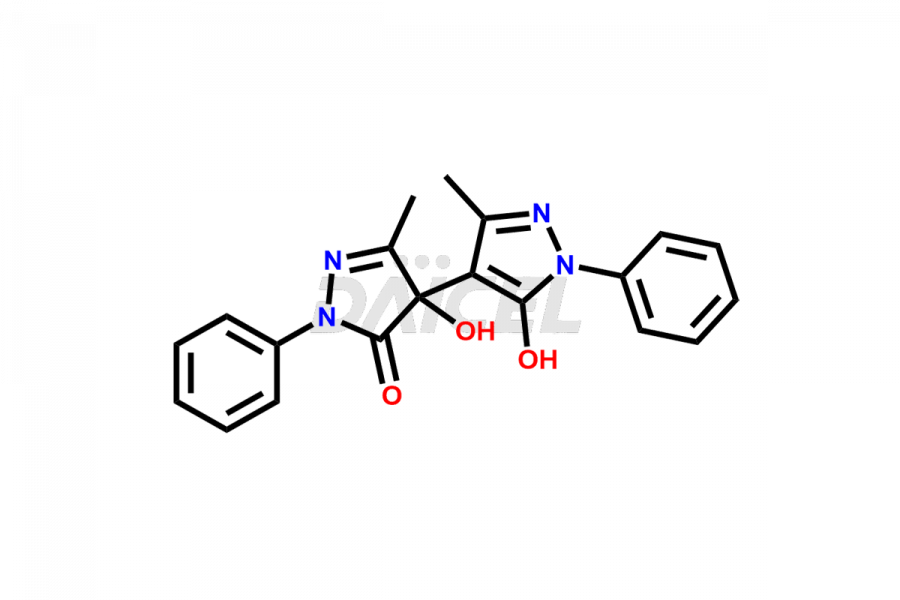
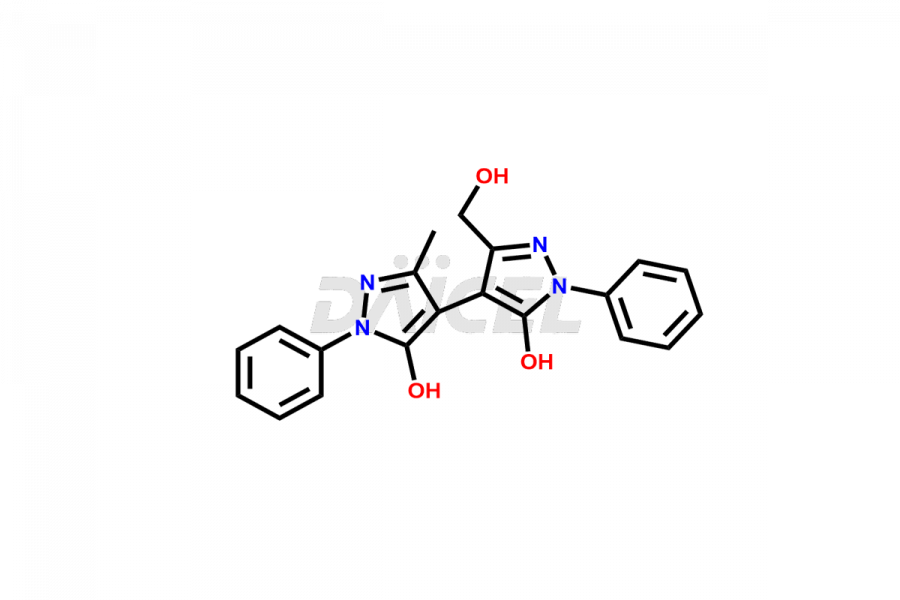
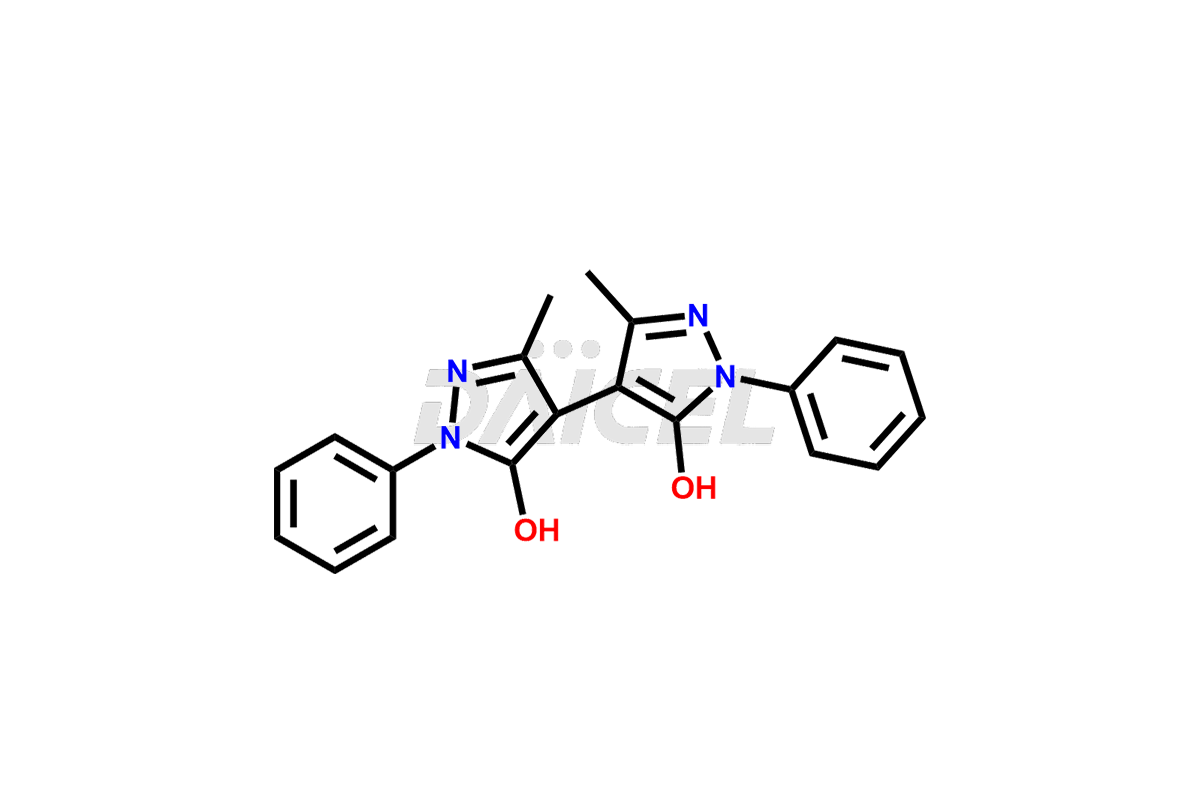
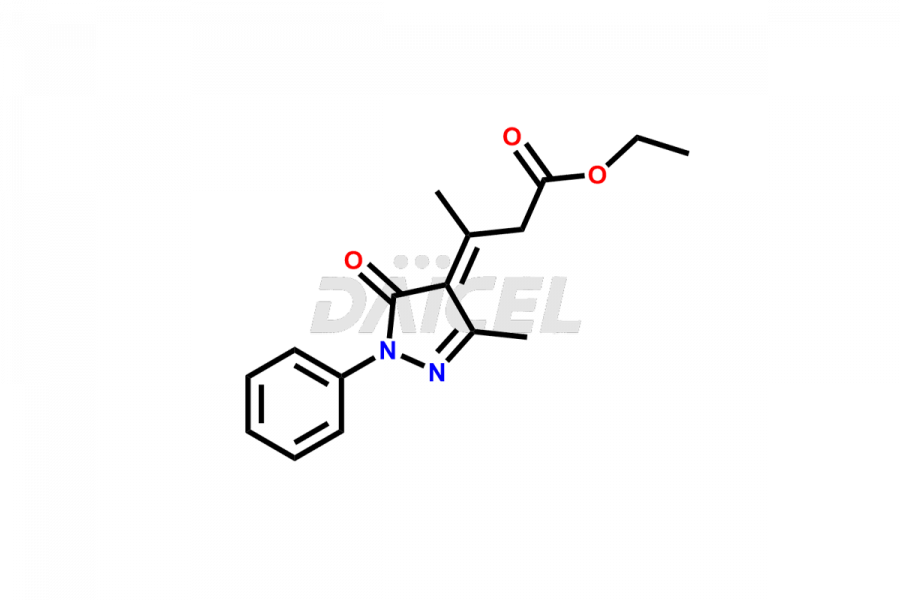
Daicel Pharma synthesizes high-quality Edaravone impurities like Edaravone Degradation Product-1, Edaravone Degradation Product-2, Edaravone Degradation Product-9, and Edaravone impurity – 6, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Edaravone. Moreover, Daicel Pharma offers custom synthesis of Edaravone impurities and delivers them globally.
Edaravone [CAS: 89-25-8] is a medicine to treat amyotrophic lateral sclerosis. It is a neuroprotective agent and free radical scavenger providing early and late-stage neuroprotection. It appears to be a suitable medicine for expanding the therapeutic time window in stroke patients. Additionally, Edaravone is also a potent antioxidant.
Edaravone: Use and Commercial Availability
Radicava is the brand name of Edaravone treating amyotrophic lateral sclerosis (ALS) in the US and Canada. It is approved in Japan for treating acute ischemic stroke.
Edaravone Structure and Mechanism of Action 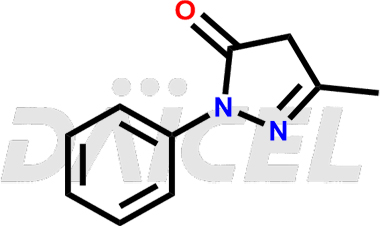
The chemical name of Edaravone is 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one. Its chemical formula is C10H10N2O, and its molecular weight is approximately 174.20 g/mol.
The mechanism of action of Edaravone is unknown concerning its therapeutic effects in patients with ALS.
Edaravone Impurities and Synthesis
During the synthesis1 of Edaravone, impurities form due to various factors such as degradation, reaction with other chemicals, and handling conditions. These impurities can affect the drug’s safety, efficacy, and quality. Therefore, it is essential to control and monitor the formation of impurities during the production and storage of Edaravone to ensure its quality and safety for patients.
Daicel provides a Certificate of Analysis (CoA) for Edaravone impurity standards, Edaravone Degradation Product-1, Edaravone Degradation Product-2, Edaravone Degradation Product-9, and Edaravone impurity – 6. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Edaravone impurity or degradation product2. We give a complete characterization report on delivery.
References
FAQ's
References
- Shuhao Zhou, Qingxia Hong, Wenliu Mei, Yan He, Chengjun Wu, and Tiemin Sun, Scale-Up of a Continuous Manufacturing Process of Edaravone, Org. Process Res. Dev. 2021, 25, 9, 2146–2153, August 27, 2021
- Madhuri Baghel, Sadhana J. Rajput, Stress degradation of edaravone: Separation, isolation and characterization of major degradation products, Biomedical Chromatography, 23 November 2017
Frequently Asked Questions
Can Edaravone impurities be produced due to the storage conditions of the drug?
Improper storage conditions of Edaravone may cause the drug to degrade and form impurities, which can affect the drug's safety and efficacy.
Are there any specific Edaravone impurities of concern?
Specific impurities in Edaravone may vary depending on the manufacturing process and the quality control specifications but are related to degradation products.
Can Edaravone impurities be eliminated?
It may not be possible to eliminate all impurities in Edaravone. However, their levels are controlled within acceptable limits by regulatory authorities.
What are the temperature conditions required to store Edaravone impurities?
Edaravone impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.