Duloxetine
General Information
Duloxetine Impurities and Duloxetine
Daicel Pharma offers high-quality impurities for Duloxetine, an active pharmaceutical ingredient. The impurity, Duloxetine Alcohol, plays a vital role in assessing the purity, reliability, and safety of Duloxetine. Daicel Pharma also offers a customized synthesis of Duloxetine impurities to cater to client requirements, with worldwide delivery options available.
Duloxetine [CAS: 116539-59-4] is a selective serotonin and norepinephrine reuptake inhibitor (SNRI). It acts as an antidepressant and for managing neuropathic pain. It is a thiophene derivative. Furthermore, it treats pain in patients with diabetes mellitus and fibromyalgia.
Duloxetine: Use and Commercial Availability
Duloxetine is a medication of the class of serotonin and norepinephrine reuptake inhibitors (SNRIs) and has diverse applications for managing various conditions. It treats major depressive disorder (MDD), generalized anxiety disorder (GAD), chronic musculoskeletal pain, diabetic peripheral neuropathy, and fibromyalgia. The drug is available under brand names such as Cymbalta, Yentreve, Drizalma Sprinkle, Nodetrip, and Ariclaim.
Duloxetine Structure and Mechanism of Action 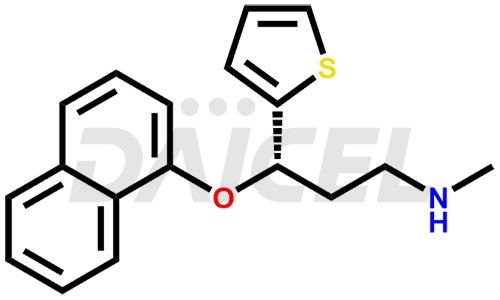
The chemical name of Duloxetine is (S)-(+)-N-Methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine. Its chemical formula is C18H19NOS, and its molecular weight is approximately 297.4 g/mol.
Duloxetine inhibits neuronal serotonin and norepinephrine uptake. It has noradrenergic activity in the CNS.
Duloxetine Impurities and Synthesis
Ensuring the quality and safety of Duloxetine drug requires diligent synthesis, analysis, and control of Duloxetine impurities. They can be unintended substances that arise during the manufacturing process1. Rigorous analytical techniques, such as high-performance liquid chromatography (HPLC), are for identifying and quantifying impurities. Strict control measures help minimize the presence of Duloxetine impurities, adhering to regulatory standards and maintaining the purity of the final product.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for the Duloxetine impurity standard, Duloxetine Alcohol. The impurity is from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Duloxetine impurities or degradation products. Duloxetine.HCl-D3, a deuterium-labeled Duloxetine compound, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
References
FAQ's
References
- Deeter, Jack; Frazier, Jeff; Staten, Gilbert; Staszak, Mike; Weigel, Leland, Asymmetric synthesis and absolute stereochemistry of LY248686, Tetrahedron Letters, Volume: 31, Issue: 49, Pages: 7101-4, 1990
- Johnson, Jason T.; Oldham, Samuel W.; Lantz, Ronald J.; DeLong, Allyn F., High performance liquid chromatographic method for the determination of Duloxetine and desmethyl duloxetine in human plasma, Journal of Liquid Chromatography & Related Technologies, Volume: 19, Issue: 10, Pages: 1631-1641, 1996
Frequently Asked Questions
Why is the analysis of Duloxetine impurities vital?
Analysis of Duloxetine impurities is vital to ensure the medication's quality, safety, and efficacy. It helps identify and quantify impurities to ensure compliance with regulatory requirements and to assess the potential impact on patient health.
Are there specific guidelines for the control of impurities in Duloxetine?
Regulatory authorities, such as the FDA and ICH, provide guidelines and recommendations for controlling impurities in pharmaceutical products, including Duloxetine. These guidelines outline acceptable limits and requirements for impurity profiling and reporting.
How are Duloxetine impurities identified and characterized?
Duloxetine impurities are identified and characterized using advanced analytical techniques such as HPLC, LC-MS, and other spectroscopic methods. These techniques help in determining the chemical structure and properties of impurities.
What temperature conditions are necessary for storing Duloxetine impurities?
Duloxetine impurities are at a controlled room temperature, specifically within 2-8 °C, or as indicated on the Certificate of Analysis (CoA) to ensure proper storage.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.



