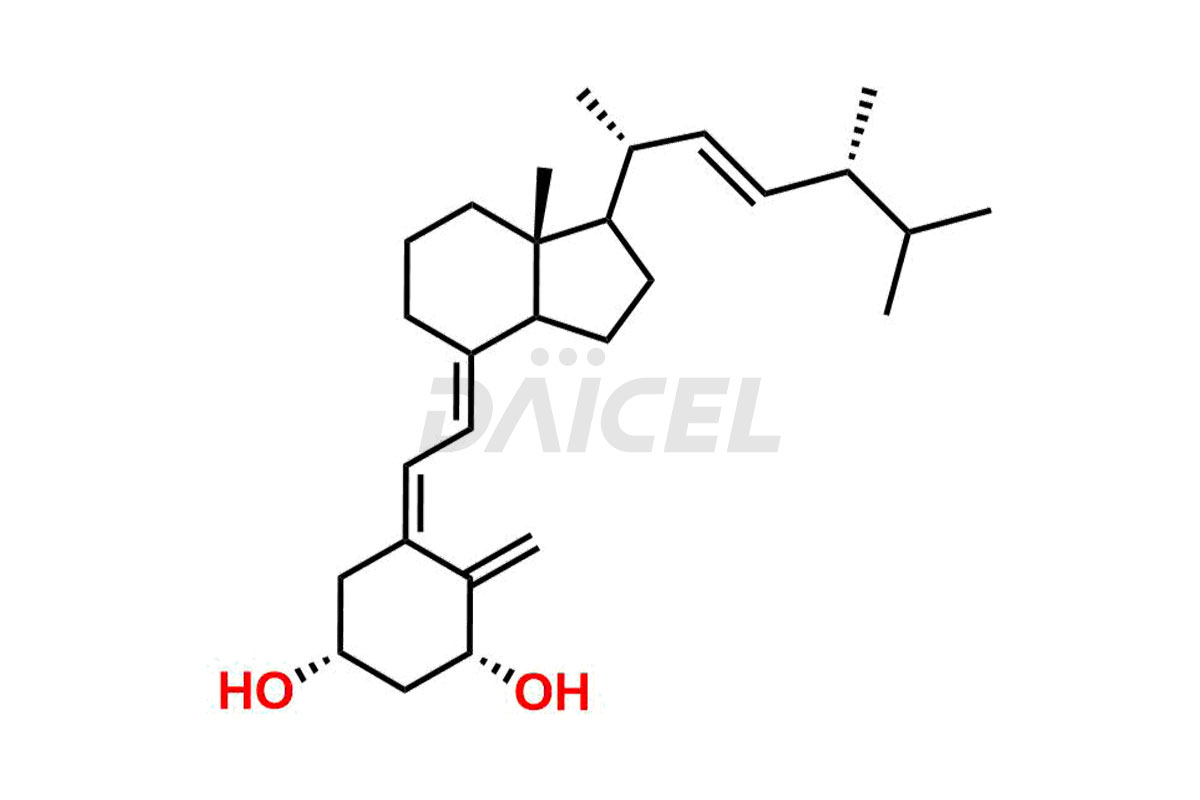
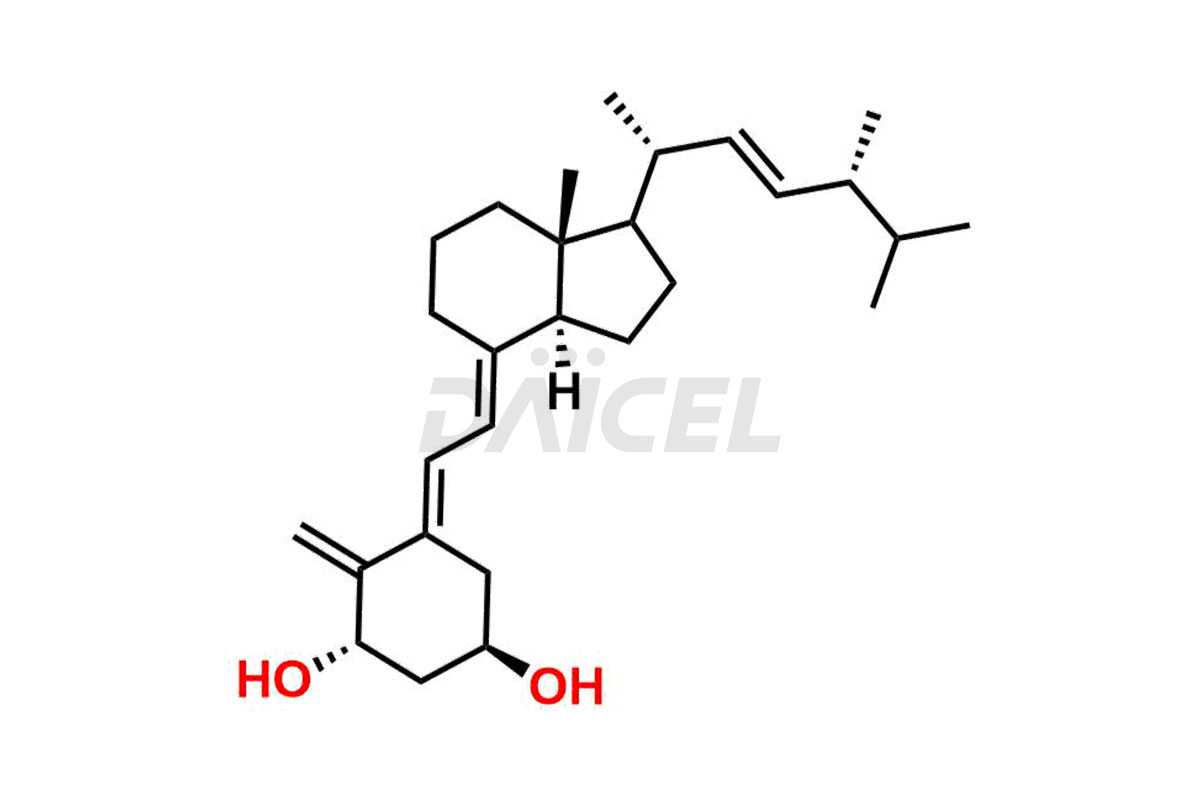
Doxercalciferol
General Information
Doxercalciferol Impurities and Doxercalciferol
Daicel Pharma offers high-quality impurities for Doxercalciferol, an active pharmaceutical ingredient. The impurities like Beta-Doxercalciferol, Pre-Doxercalciferol, and Trans-Doxercalciferol, play a vital role in assessing the purity, reliability, and safety of Doxercalciferol. Daicel Pharma also offers a customized synthesis of Doxercalciferol impurities to cater to client requirements, with worldwide delivery options available.
Doxercalciferol [CAS: 54573-75-0], a synthetic vitamin D analog, treats secondary hyperparathyroidism in patients with chronic kidney disease. It suppresses the excess parathyroid hormone (PTH) production that controls blood calcium levels. Doxercalciferol acts as a provitamin, promoting the synthesis of active hormone forms, and also aids in preserving bone density. It belongs to the vitamin D family and is a hydroxy secosteroid.
Doxercalciferol: Use and Commercial Availability
Doxercalciferol helps manage secondary hyperparathyroidism in two patient populations: those with chronic kidney disease undergoing dialysis and those with Stage 3 or Stage 4 chronic kidney disease. The drug is available under the brand name Hectorol.
Doxercalciferol Structure and Mechanism of Action 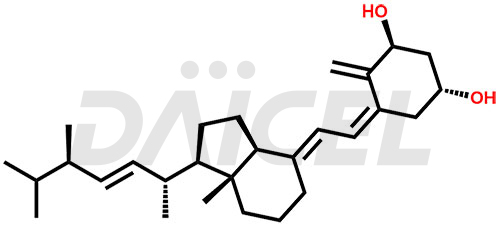
The chemical name of Doxercalciferol is 1R,3S,5Z)-4-Methylene-5-[(2E)-2-[(1R,3aS,7aR)-octahydro-7a-methyl-1-[(1R,2E,4R)-1,4,5-trimethyl-2-hexen-1-yl]-4H-inden-4-ylidene]ethylidene]-1,3-cyclohexanediol. Its chemical formula is C28H44O2, and its molecular weight is approximately 412.6 g/mol.
Doxercalciferol converts to its biologically active Vitamin D metabolites in the liver. It controls the intestinal absorption of dietary calcium and mobilizes calcium from the skeleton in conjunction with parathyroid hormone (PTH).
Doxercalciferol Impurities and Synthesis
The synthesis, analysis, and control of Doxercalciferol impurities are crucial for ensuring the quality and safety of the Doxercalciferol drug. Impurities in Doxercalciferol form during the manufacturing process1 as unintended byproducts or related substances. Rigorous analytical techniques, such as high-performance liquid chromatography (HPLC), help identify and quantify these impurities. Strict control measures help minimize the presence of Doxercalciferol impurities, adhering to regulatory standards and maintaining the purity of Doxercalciferol.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Doxercalciferol impurity standards, including Beta-Doxercalciferol, Pre-Doxercalciferol, and Trans-Doxercalciferol. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We provide additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Doxercalciferol impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Lam, H. Y.; Schnoes, H. K.; DeLuca, H., 1α-Hydroxy vitamin D2. Potent synthetic analog of vitamin D2, Science (Washington, DC, United States), Volume: 186, Issue: 4168, Pages: 1038-40, 1974
- Simonzadeh, Ninus; Ronsen, Bruce; Upadhyaya, Subhash; Wilkinson, Erik; Kanesvaran, Kiran; Patel, Vijay; Bendale, Pravin, Determination of Degradation Products of Doxercalciferol by Solid-Phase Extraction and Reversed-Phase HPLC, Journal of Chromatographic Science, Volume: 52, Issue: 6, Pages: 520-525, 2014
Frequently Asked Questions
How are Doxercalciferol impurities identified and characterized during the development phase?
During the development phase, Doxercalciferol impurities are identified and characterized using analytical techniques, such as mass spectrometry, elemental analysis, etc.
How do regulatory authorities monitor and enforce control over Doxercalciferol impurities?
Regulatory authorities conduct inspections, review documentation, and perform sampling and testing of Doxercalciferol to ensure compliance with impurity control guidelines and regulations.
What solvent helps in analyzing Doxercalciferol impurities?
Acetonitrile is the solvent used for analyzing many impurities in Doxercalciferol.
What are the recommended temperature conditions for storing Doxercalciferol impurities?
It is advisable to store Doxercalciferol impurities at a controlled room temperature within the range of 2-8 °C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.