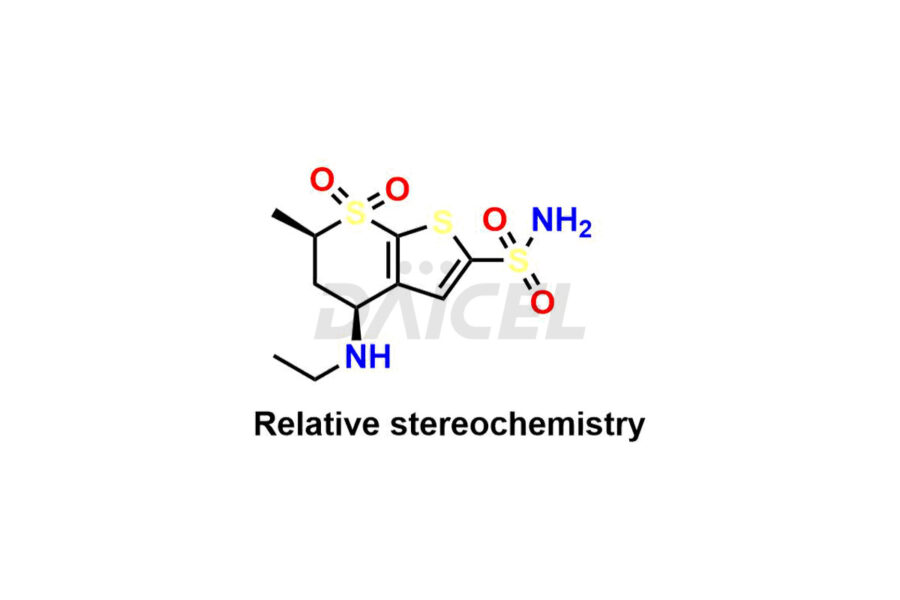
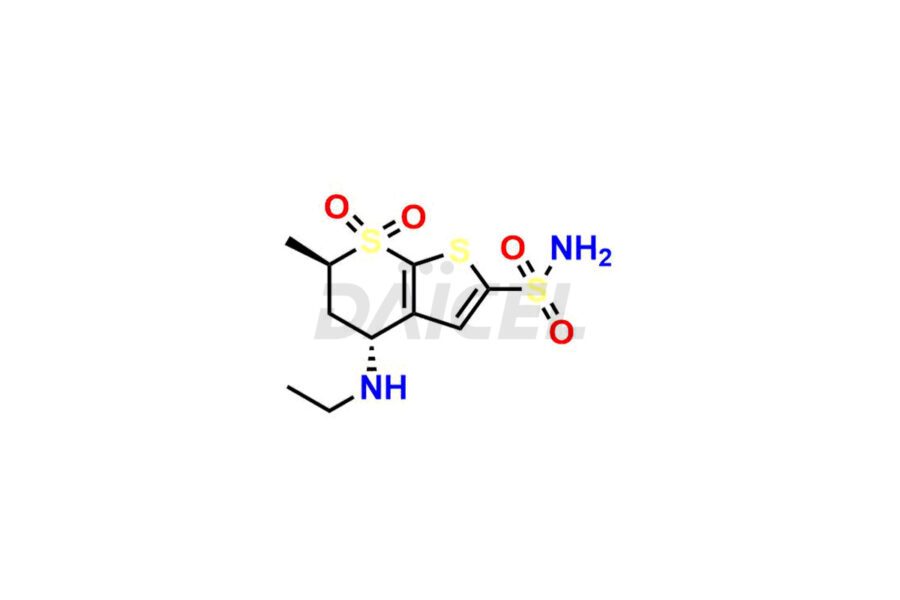
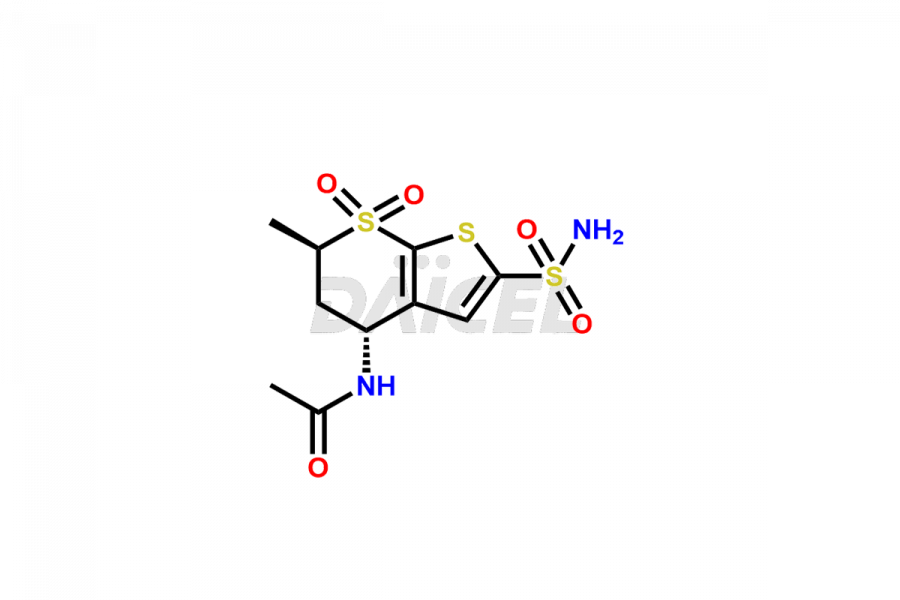
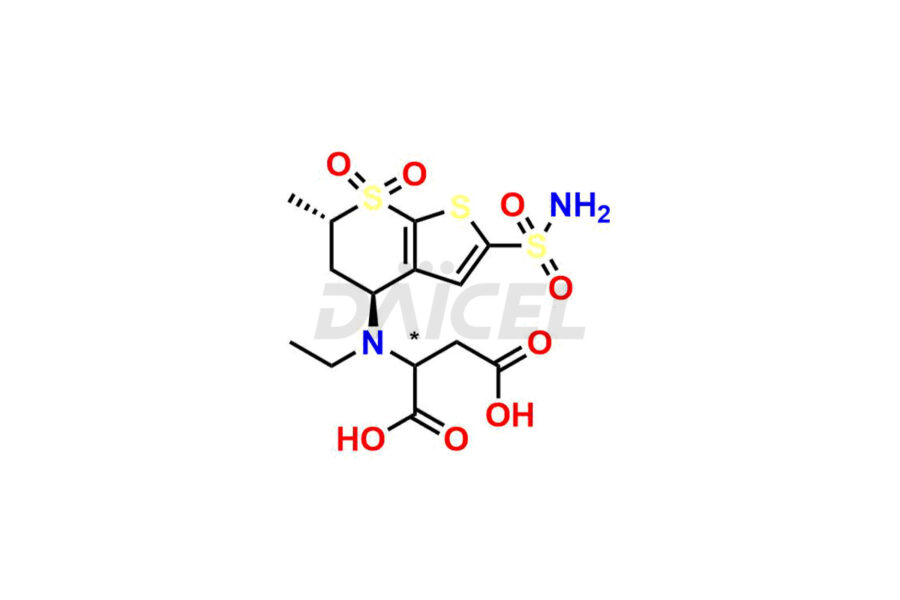
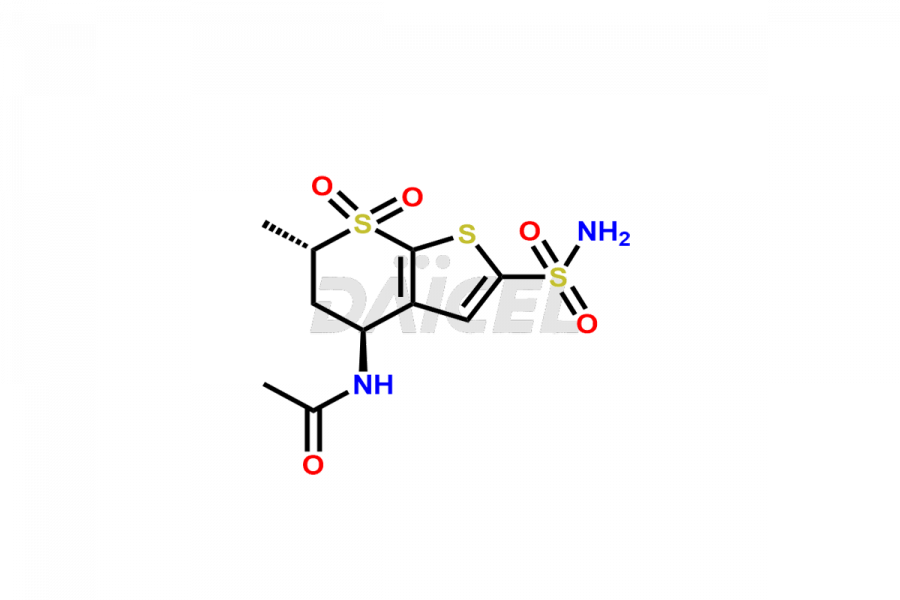
Dorzolamide
General Information
Dorzolamide Impurities and Dorzolamide
Daicel Pharma specializes in offering high-quality impurities for Dorzolamide, an active pharmaceutical ingredient. These impurities, including Dorzolamide Diastereomers, Dorzolamide Enantiomer, Dorzolamide intermediate Diastereomers, Dorzolamide Intermediate Enantiomer, Dorzolamide Maleic acid adduct, and Dorzolamide N-Desethyl N-Acetyl Analog, play a vital role in assessing the purity, reliability, and safety of Dorzolamide. Daicel Pharma also offers a customized synthesis of Dorzolamide impurities to cater to client requirements, with worldwide delivery options available.
Dorzolamide [CAS: 120279-96-1] is an antiglaucoma drug that acts as a carbonic anhydrase inhibitor. It lowers intraocular pressure in the treatment of open-angle glaucoma. Additionally, Dorzolamide serves as an antihypertensive agent.
Dorzolamide: Use and Commercial Availability
Dorzolamide, available under Trusopt, is a second-generation inhibitor of carbonic anhydrase II that treats glaucoma. Dorzolamide lowers intraocular pressure by reducing the production of aqueous humor. It is formulated as a 2% eyedrop and effectively reduces intraocular pressure (IOP). Patients with open-angle glaucoma or ocular hypertension can benefit from Dorzolamide’s IOP-lowering effects.
Dorzolamide Structure and Mechanism of Action 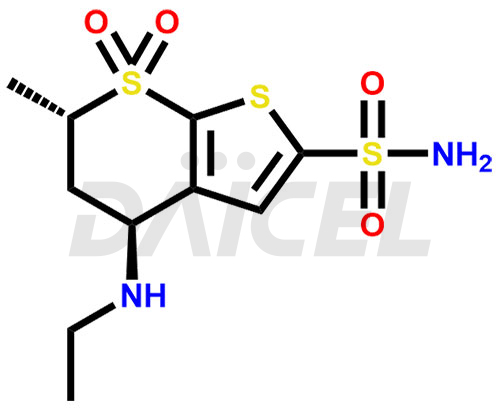
The chemical name of Dorzolamide is (4S,6S)-4-(Ethylamino)-5,6-dihydro-6-methyl-4H-thieno-[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide. Its chemical formula is C10H16N2O4S3, and its molecular weight is approximately 324.4 g/mol.
Dorzolamide inhibits human carbonic anhydrase II in the ciliary processes of the eye and decreases aqueous humor secretion.
Dorzolamide Impurities and Synthesis
The formation of impurities in Dorzolamide can impact its quality, safety, and effectiveness. Impurities can arise during the synthetic process1 or storage of Dorzolamide and may include degradation products, residual solvents, or related substances. It is necessary to synthesize and characterize these impurities to understand their nature and potential impact. Analytical methods, such as chromatography and spectroscopy, help analyze and quantify impurities in Dorzolamide. Implementation of strict control measures helps establish acceptable limits for impurity levels, ensuring the quality and purity of Dorzolamide for safe and effective use in patients with glaucoma.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Dorzolamide impurity standards, including Dorzolamide Diastereomers, Dorzolamide Enantiomer, Dorzolamide intermediate Diastereomers, Dorzolamide Intermediate Enantiomer, Dorzolamide Maleic acid adduct, and Dorzolamide N-Desethyl N-Acetyl Analog. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Dorzolamide impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Baldwin, John J.; Ponticello, Gerald S.; Christy, Marcia E., Antiglaucoma thieno-thiopyran and thieno-thiepin sulfonamide derivatives, compositions, and method of use thereof, Merck and Co., Inc., United States, US4677115A, June 30, 1987
- Constanzer, M. L.; Chavez, C. M.; Matuszewski, B. K., Low level determination of dorzolamide and its de-ethylated metabolite in human plasma by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry, Journal of Pharmaceutical and Biomedical Analysis, Volume: 15, Issue: 7, Pages: 1001-1008, 1997
Frequently Asked Questions
Can Dorzolamide impurities be removed or reduced during the manufacturing process?
Efforts are made during the manufacturing process of Dorzolamide to minimize impurity formation. Techniques such as purification, filtration, and optimization of reaction conditions help to remove or reduce impurities. Further, appropriate storage and packaging practices can maintain the drug's quality and minimize impurity formation.
Are there specific impurities in Dorzolamide?
The common impurities in Dorzolamide include related compounds formed during the synthetic process, degradation products resulting from exposure to light, heat, or moisture, and residual solvents used in manufacturing.
Which solvent helps in the analysis of Dorzolamide impurities?
Methanol is a solvent used in analyzing many impurities in Dorzolamide.
What are the temperature conditions required to store Dorzolamide impurities?
Dorzolamide impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.