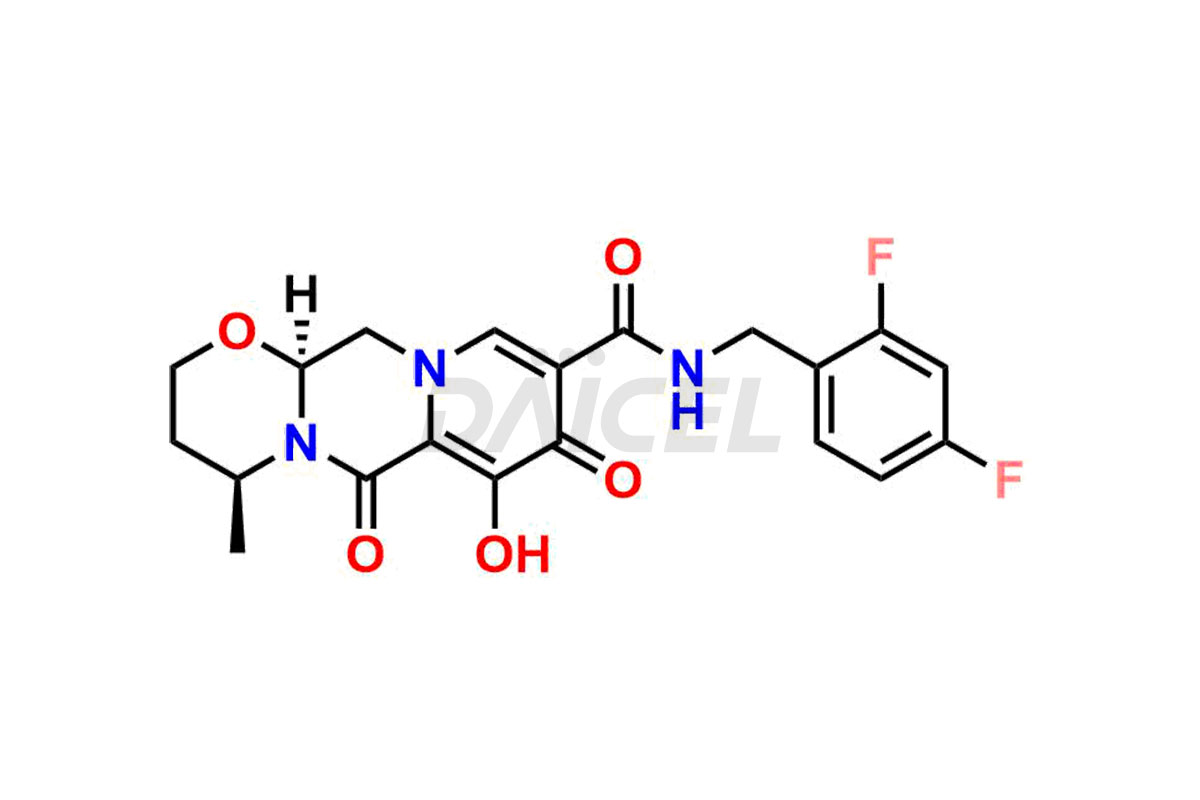
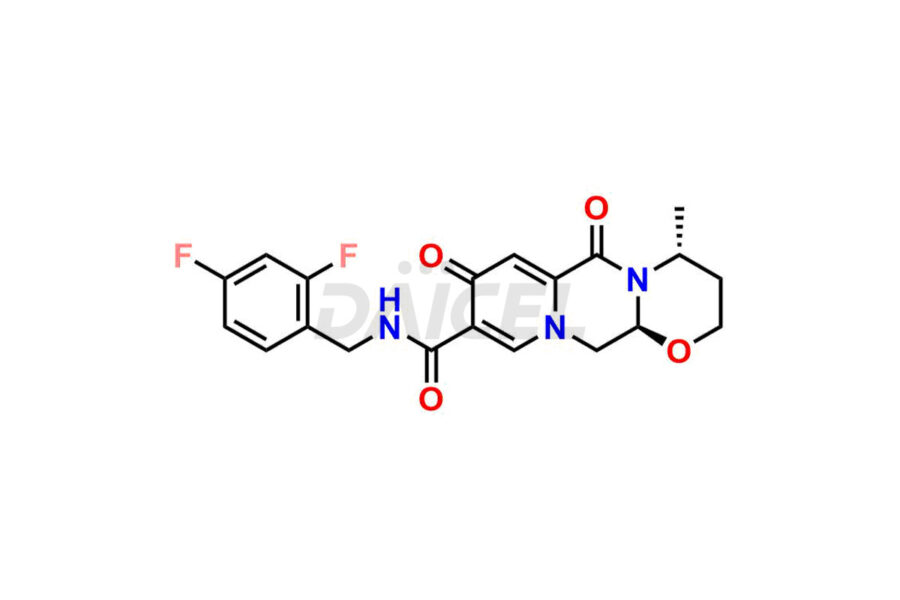
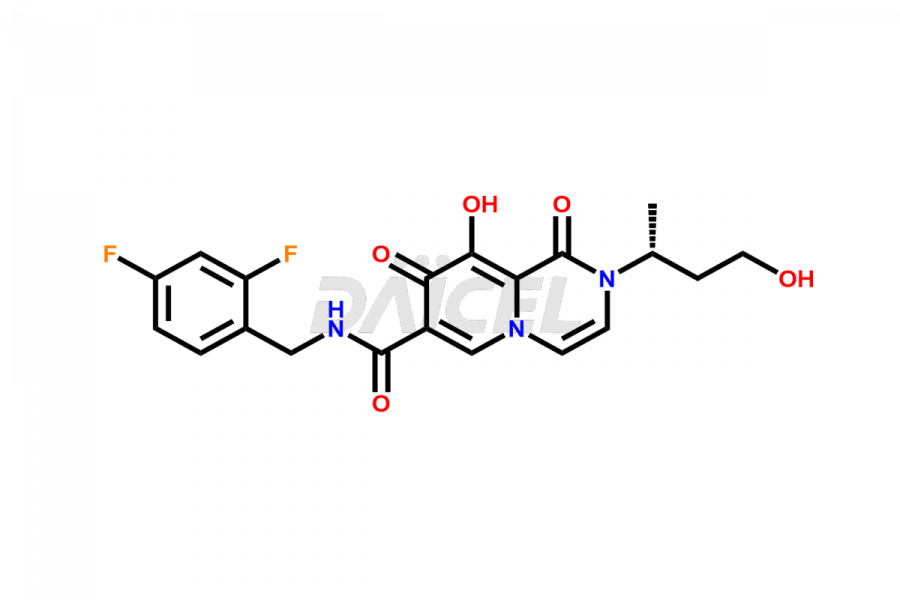
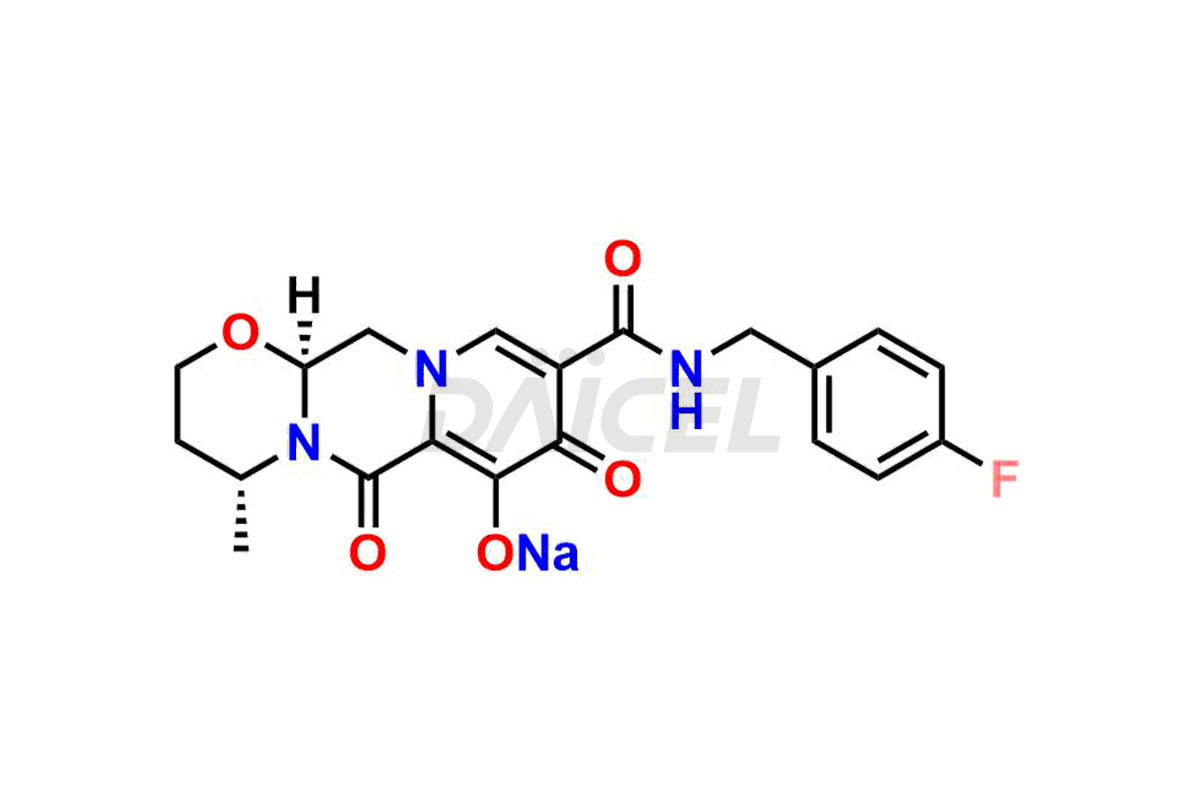
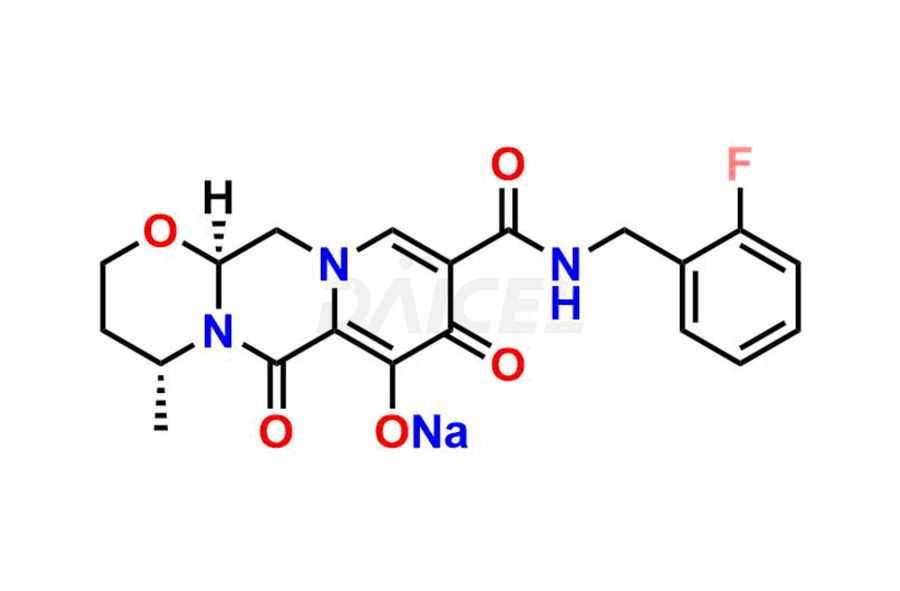
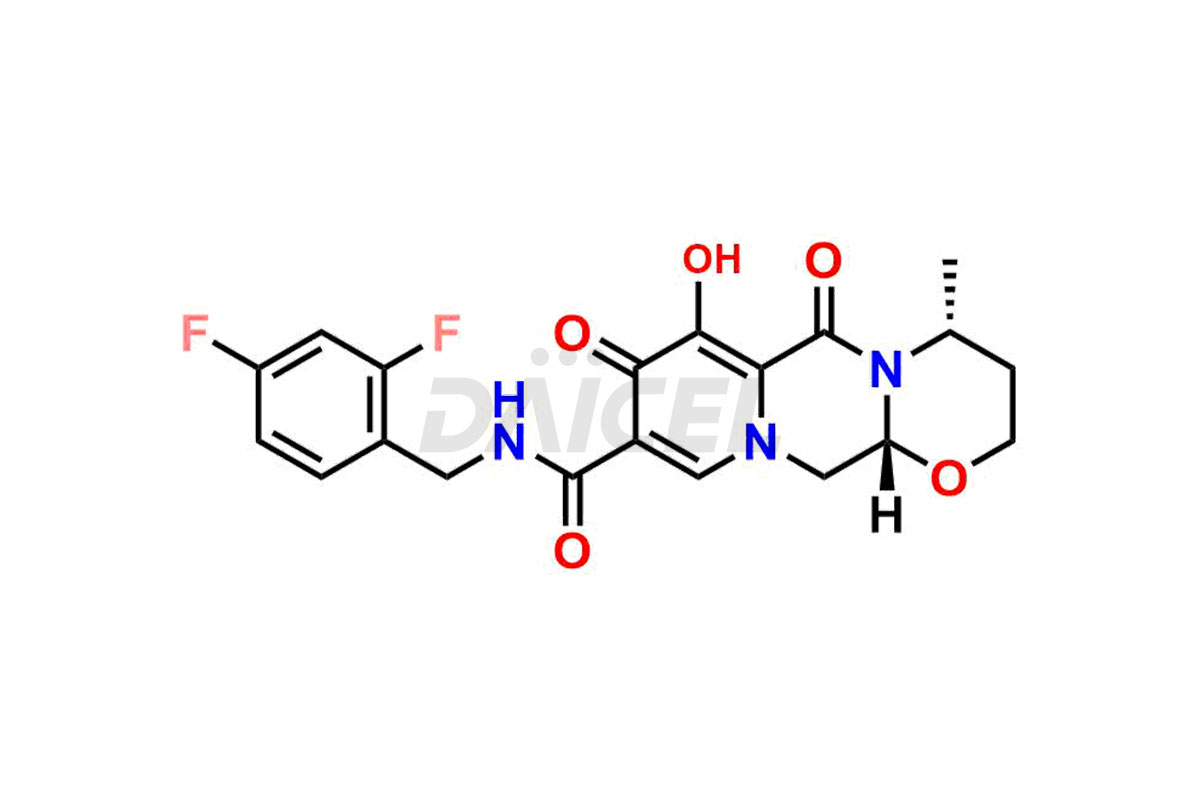
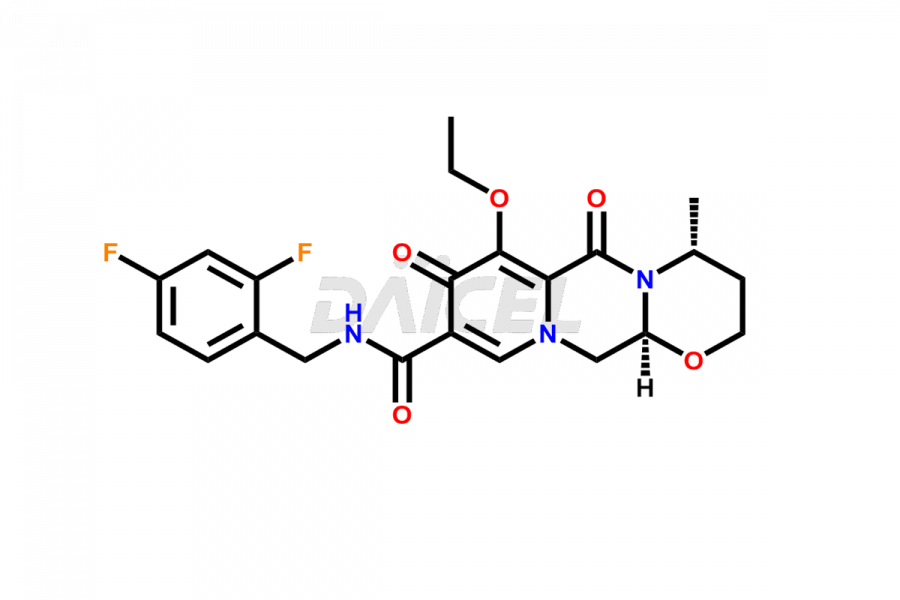
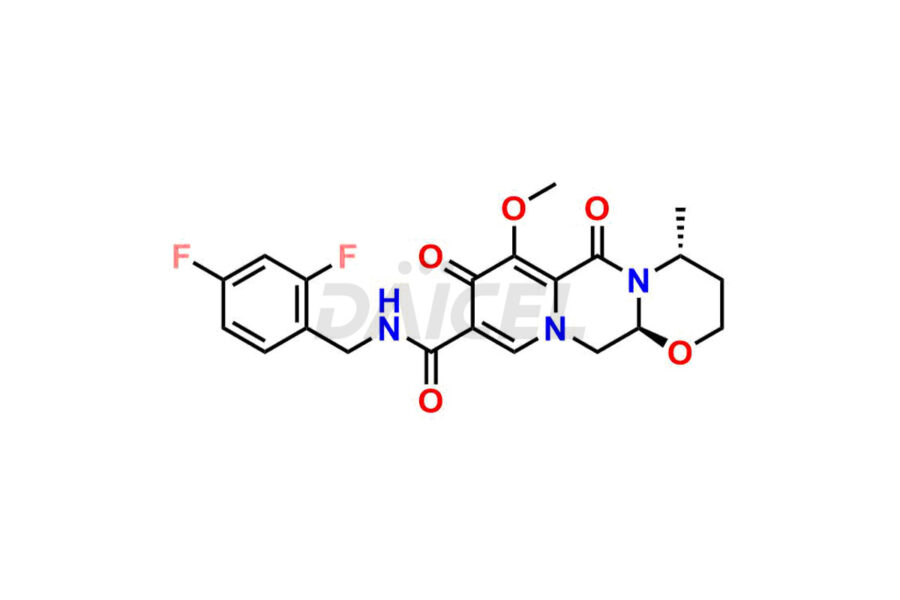
Dolutegravir
General Information
Dolutegravir Impurities and Dolutegravir
Daicel Pharma specializes in offering high-quality impurities for Dolutegravir, an active pharmaceutical ingredient. These impurities, including (S, R)-Dolutegravir Isomer, (S, S)-Dolutegravir Isomer (Dolutegravir Impurity-16), Des Hydroxy Dolutegravir, Dolutegravir Impurity E, Dolutegravir Impurity-4, Dolutegravir Impurity-5, Dolutegravir R, R isomer, O ethyl Dolutegravir, and O-Methyl Dolutegravir, play a vital role in assessing the purity, reliability, and safety of Dolutegravir. Daicel Pharma also offers a customized synthesis of Dolutegravir impurities to cater to clients’ requirements, with worldwide delivery options available.
Dolutegravir [CAS: 1051375-16-6], an integrase strand-transfer inhibitor (INSTI), is an orally bioavailable drug that targets HIV-1 infection. It treats HIV infections with other antiretroviral agents.
Dolutegravir: Use and Commercial Availability
Dolutegravir is an antiretroviral agent that treats HIV-1 infections. The US FDA has approved the combination therapy of Dolutegravir and [rilpivirine] for adults with suppressed HIV-1 virus levels and a stable regimen for at least six months. Dolutegravir is also available with [lamivudine] and [abacavir] for treating HIV-1 in adult and pediatric patients weighing ≥10kg. The drug is available under the brand names, Dovato, Tivicay, and Tivicay PD.
Dolutegravir Structure and Mechanism of Action 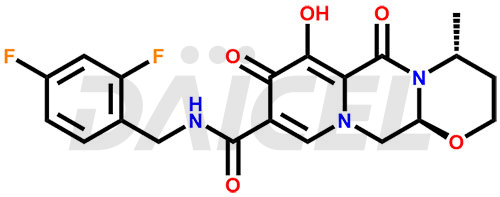
The chemical name of Dolutegravir is (4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide. Its chemical formula is C20H19F2N3O5, and its molecular weight is approximately 419.4 g/mol.
Dolutegravir binds to the integrase active site by inhibiting HIV integrase. It blocks the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration and prevents the HIV replication cycle.
Dolutegravir Impurities and Synthesis
The presence of impurities in Dolutegravir, an integrase strand-transfer inhibitor used for treating HIV-1 infection, can affect its safety, efficacy, and stability. Impurities may arise during the synthesis1 or storage of Dolutegravir and can be related to starting materials, intermediates, or degradation products. It is crucial to synthesize and analyze these impurities to ensure the quality and purity of the drug. Analytical methods, chromatography, and spectroscopy will help detect and quantify impurities. Implementing strict control measures helps establish acceptance criteria for impurity levels, ensuring that Dolutegravir meets regulatory requirements and remains safe for patient use.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Dolutegravir impurity standards, including (S, R)-Dolutegravir Isomer, (S, S)-Dolutegravir Isomer (Dolutegravir Impurity-16), Des Hydroxy Dolutegravir, Dolutegravir Impurity E, Dolutegravir Impurity-4, Dolutegravir Impurity-5, Dolutegravir R, R isomer, O ethyl Dolutegravir, and O-Methyl Dolutegravir. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Dolutegravir impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Johns, Brian Alvin; Kawasuji, Takashi; Taishi, Teruhiko; Taoda, Yoshiyuki, Bicyclic Carbamoylpyridone Derivative Having HIV Integrase Inhibiting Activity, Shionogi & Co., Ltd., Japan, EP1852434B1, July 13, 2011
- Bennetto-Hood, Chantelle; Tabolt, Glenn; Savina, Paul; Acosta, Edward P., A sensitive HPLC-MS/MS method for the determination of Dolutegravir in human plasma, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 945-946, Pages: 225-232, 2014
Frequently Asked Questions
What methods help in the analysis of Dolutegravir impurities?
Various analytical methods such as chromatography (HPLC, LC), spectroscopy (UV, IR), and mass spectrometry help analyze Dolutegravir impurities. These methods help detect, quantify, and characterize impurities present in the drug substance.
How are impurities controlled in the manufacturing of Dolutegravir?
Strict control measures are implemented during the manufacturing process of Dolutegravir to minimize the formation of impurities. It includes optimizing reactions, using high-quality starting materials, and implementing proper storage and handling conditions to prevent degradation.
Can Dolutegravir impurities affect the drug's safety and efficacy?
Yes, impurities in Dolutegravir can potentially impact its safety and efficacy. Depending on the nature and concentration of impurities, they may introduce toxicity, reduce drug stability, or interfere with the intended therapeutic action of Dolutegravir.
What are the temperature conditions required to store Dolutegravir impurities?
Dolutegravir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.