Dobutamine
General Information
Dobutamine Impurities and Dobutamine
Daicel Pharma specializes in offering high-quality impurities for Dobutamine, a crucial active pharmaceutical ingredient. These impurities, including Dobutamine (R) Isomer Hydrochloride and Dobutamine (S) Isomer Hydrochloride, play a vital role in assessing Dobutamine purity, reliability, and safety. Daicel Pharma also offers a customized synthesis of Dobutamine impurities to cater to clients’ requirements, with worldwide delivery options available.
Dobutamine [CAS: 34368-04-2], a synthetic catecholamine, acts as a sympathomimetic compound. It targets beta-1 adrenergic receptors. It is a cardiotonic agent. This drug plays multiple roles, functioning as a cardiotonic agent, sympathomimetic agent, and beta-adrenergic agonist.
Dobutamine: Use and Commercial Availability
Dobutamine is approved by the US FDA for short-term use in patients with heart failure or cardiac decompensation caused by cardiac surgical procedures, leading to decreased contractility. Dobutamine causes vasodilation by stimulating beta-2 adrenergic receptors in blood vessels. It treats hospitalized patients with severe systolic dysfunction. The brand name under which the drug is available is Dobutrex.
Dobutamine Structure and Mechanism of Action 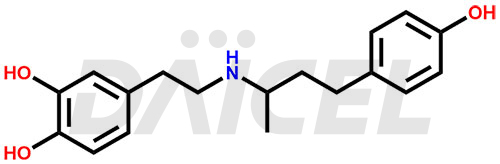
The chemical name of Dobutamine is 4-[2-[[3-(4-Hydroxyphenyl)-1-methylpropyl]amino]ethyl]-1,2-benzenediol. Its chemical formula is C18H23NO3, and its molecular weight is approximately 301.4 g/mol.
Dobutamine has inotropic effects on the myocardium due to the activation of the beta-1 receptors.
Dobutamine Impurities and Synthesis
Impurities in Dobutamine form due to various factors such as synthesis1, storage conditions, or chemical interactions. Analyzing and controlling these impurities ensures drug quality, safety, and efficacy. Impurity analysis plays a crucial role in identifying, quantifying, and characterizing these unwanted substances, enabling the establishment of specific standards and limits. Stringent control measures are necessary to comply with regulatory requirements, maintain product stability, and minimize potential risks associated with impurities in Dobutamine.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Dobutamine impurity standards, including Dobutamine (R) Isomer Hydrochloride and Dobutamine (S) Isomer Hydrochloride. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Dobutamine impurities or degradation products. Every delivery accompanies a complete characterization report.
References
FAQ's
References
- Tuttle, Ronald R.; Mills, Jack, Method for increasing cardiac contractility, Eli Lilly and Co., United States, US3987200A, October 19, 1976
- McKennon, D. W.; Kates, R. E., High-pressure liquid chromatographic determination of plasma dobutamine concentrations, Journal of Pharmaceutical Sciences, Volume: 67, Issue: 12, Pages: 1756-7, 1978
Frequently Asked Questions
What are the potential sources of Dobutamine impurities?
The potential sources of Dobutamine impurities are starting materials, reagents, catalysts, solvents, and degradation products.
Can impurity analysis help identify the source or root cause of impurities in Dobutamine?
Impurity analysis can provide valuable insights into their source or root cause, aiding process optimization and control.
Which solvent helps in the analysis of Dobutamine impurities?
Acetonitrile and water solution, in a 1:1 ratio, help analyze many impurities in Dobutamine.
What are the temperature conditions required to store Dobutamine impurities?
Dobutamine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.



