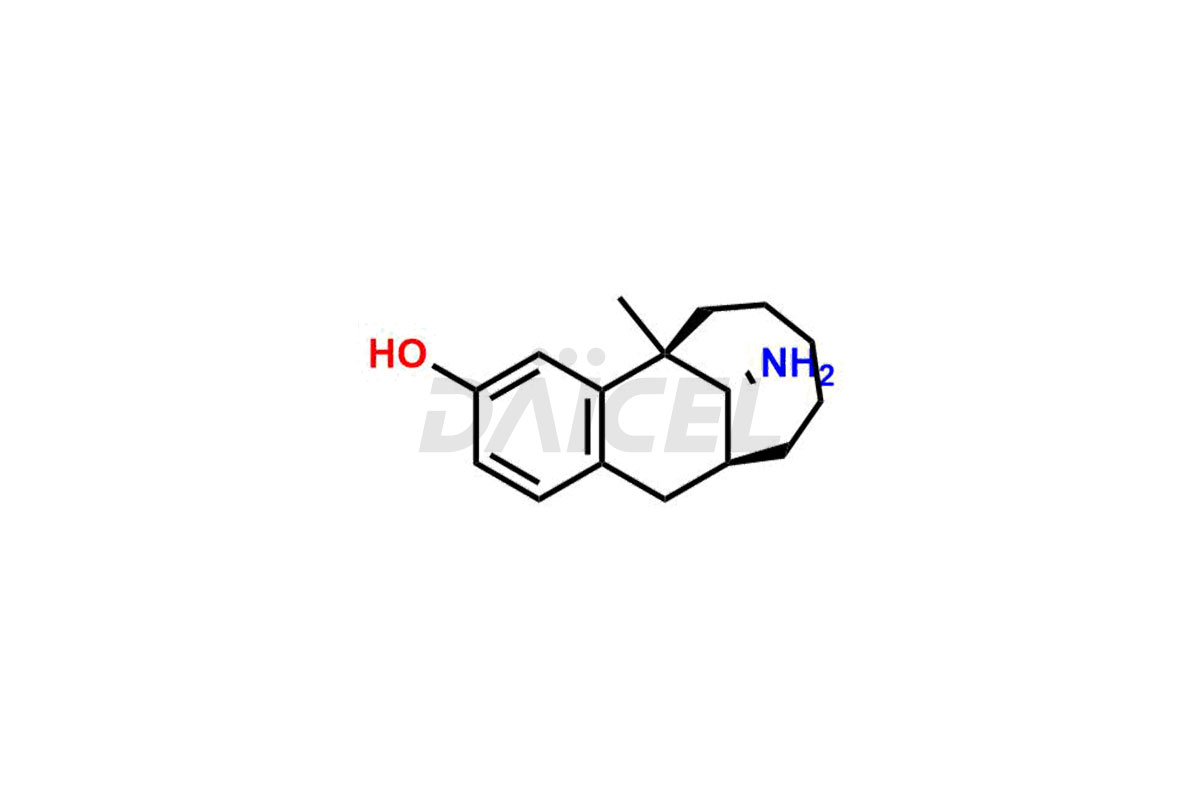
Dezocine
General Information
Dezocine Impurities and Dezocine
Daicel Pharma synthesizes Dezocine impurities of exceptional quality, such as (5R,11S,13R)-13-amino-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methanobenzo[10]annulen-3-ol and (5R,11S,13R)-3-methoxy-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methanobenzo[10]annulen-13-amine. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Dezocine. Besides, Daicel Pharma provides custom synthesis of Dezocine impurities to meet clients’ demands for delivery worldwide.
Dezocine [CAS: 53648-55-8], a synthetic opioid analgesic, exhibits a combination of opioid agonist and antagonist properties. While it is for pain management, its administration to individuals already dependent on other opioids can result in an opioid withdrawal syndrome.
Dezocine: Use and Commercial Availability
Dezocine, available under the brand, Dalgan, treats moderate to severe pain. It belongs to the class of partial agonist opioids, with buprenorphine, which has a limited role in managing cancer pain due to the ceiling effect for analgesia. Structurally, Dezocine is a bridged aminotetralin, sharing similarities with other opioid agonist/antagonist drugs like pentazocine and butorphanol. As a result, it interacts with similar targets within the body. Dezocine’s analgesic and somnolent effects are dose-dependent, indicating its action on the kappa-opioid receptor.
Dezocine Structure and Mechanism of Action 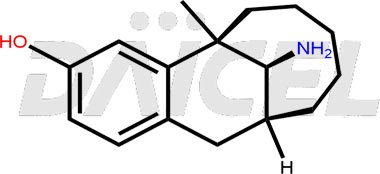
The chemical name of Dezocine is (5R,11S,13S)-13-Amino-5,6,7,8,9,10,11,12-octahydro-5-methyl-5,11-methanobenzocyclodecen-3-ol. Its chemical formula is C16H23NO, and its molecular weight is approximately 245.36 g/mol.
Dezocine binds to various opiate receptors.
Dezocine Impurities and Synthesis
During the synthesis1 of Dezocine, impurities form as byproducts or reaction intermediates. They can arise from the starting materials, reagents, or reaction conditions used in manufacturing. It is necessary to identify and characterize them to ensure the purity and safety of the final Dezocine product. Analytical techniques such as chromatography and spectroscopy help in impurity profiling and quantification. Manufacturers employ strict quality control measures to minimize impurities and adhere to regulatory guidelines, ensuring the production of high-quality Dezocine for therapeutic use.
Daicel Pharma offers a Certificate of Analysis (CoA) for Dezocine impurity standards, such as (5R,11S,13R)-13-amino-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methanobenzo[10]annulen-3-ol and (5R,11S,13R)-3-methoxy-5-methyl-5,6,7,8,9,10,11,12-octahydro-5,11-methanobenzo[10]annulen-13-amine, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Dezocine impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Freed, Meier E.; Potoski, John R., Benzobicycloalkane Compounds, American Home Products Corp., United States, GB1363658A, August 14, 1974
- Wilson, J. M.; Cohen, R. I.; Kezer, E. A.; Smith, E. R., Analysis of dezocine in serum and urine by high performance liquid chromatography and pre-column derivatization, Journal of Liquid Chromatography, Volume: 17, Issue: 19, Pages: 4245-57, 1994
Frequently Asked Questions
How are impurities characterized and identified in Dezocine?
Impurities in Dezocine are characterized and identified using advanced analytical techniques such as mass spectrometry, chromatography, and spectroscopy. Comparisons are made to reference standards and impurity libraries to determine the nature and structure of impurities.
How is the control of Dezocine impurities ensured throughout its shelf life?
The control of impurities in Dezocine throughout its shelf life is through stability studies and ongoing quality control testing. These studies monitor the degradation and their formation over time to ensure that the drug remains within acceptable limits throughout its designated shelf life.
Which solvent helps in the analysis of Dezocine impurities?
Acetonitrile is a solvent used in analyzing many impurities in Dezocine.
What are the temperature conditions required to store Dezocine impurities?
Dezocine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.