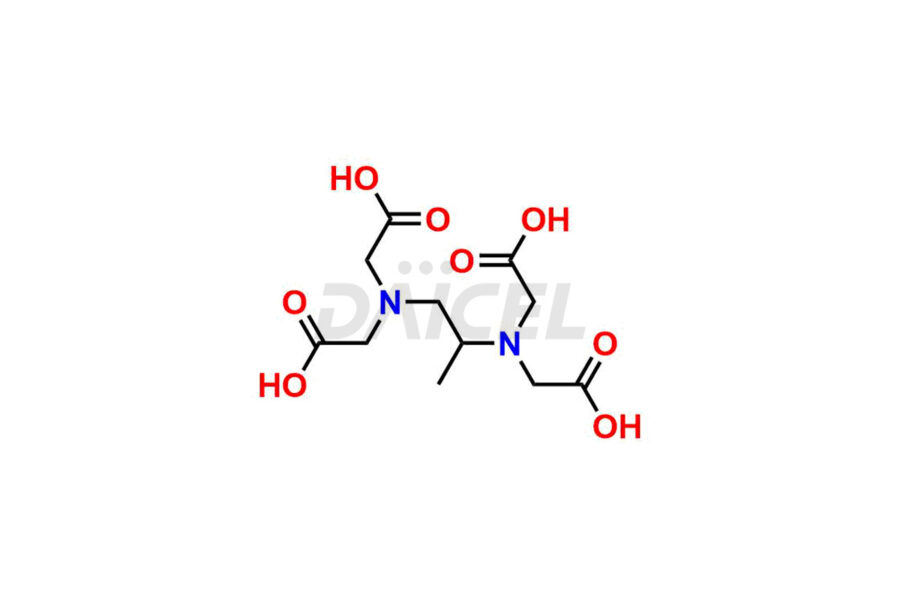
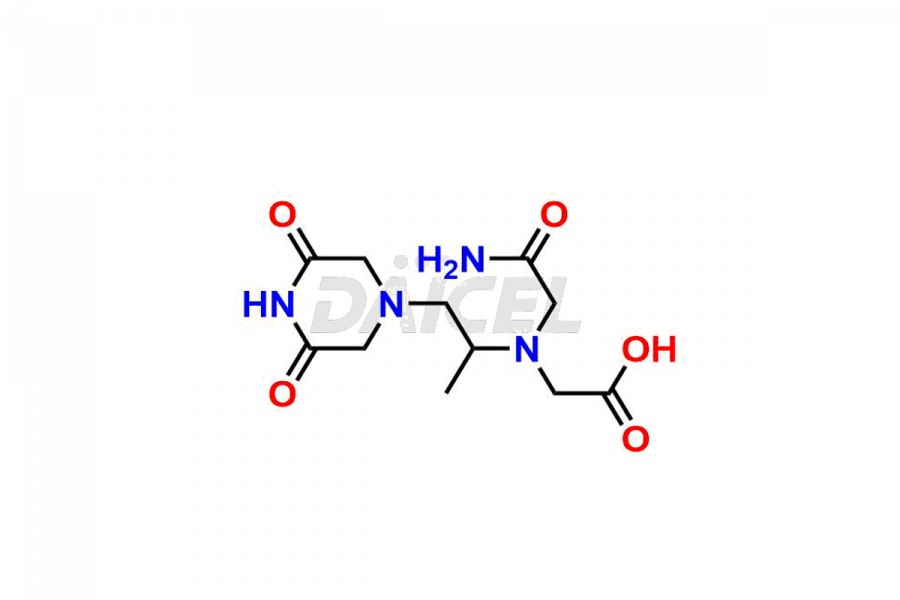
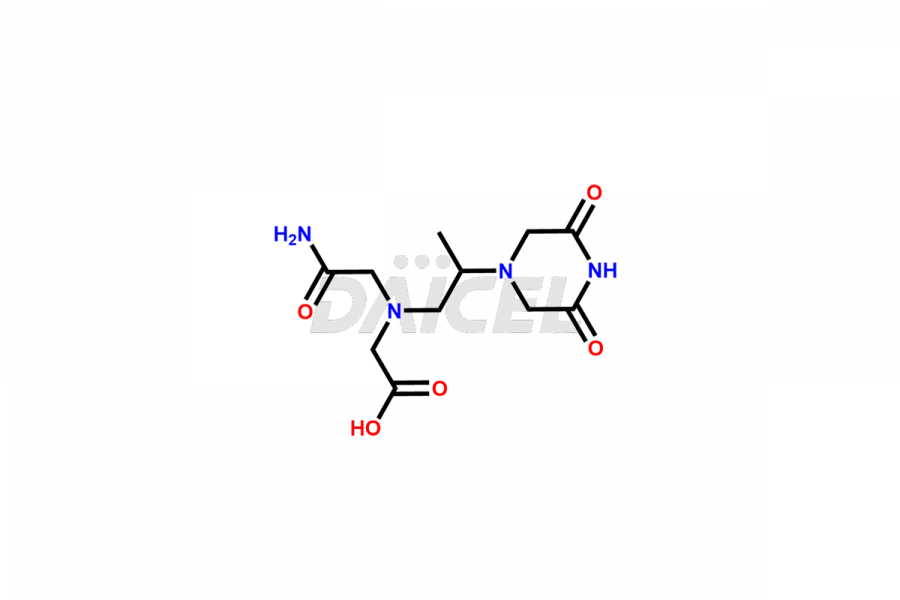
Dexrazoxane
General Information
Dexrazoxane Impurities and Dexrazoxane
Daicel Pharma synthesizes Dexrazoxane impurities of exceptional quality, such as Dexrazoxane Impurity-A, Dexrazoxane Impurity-C, and Dexrazoxane Impurity-D. These impurities are crucial to assess the purity, reliability, and safety of Dexrazoxane, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Dexrazoxane impurities to meet clients’ demands for delivery worldwide.
Dexrazoxane [CAS: 24584-09-6], the (+)-enantiomorph of razoxane, exhibits iron-chelating, chemoprotective, cardioprotective, and antineoplastic properties. It acts as an antimitotic agent while also possessing immunosuppressive effects. Dexrazoxane provides cardioprotection against anthracycline toxicity by inhibiting the formation of a toxic iron-anthracycline complex.
Dexrazoxane: Use and Commercial Availability
Dexrazoxane, an FDA-approved cardioprotective drug, has demonstrated its effectiveness in managing cardiac toxicity associated with anthracycline-based chemotherapy in cancer patients, particularly those with advanced breast cancer, soft tissue sarcomas, or small-cell lung cancer. It treats tissue damage caused by anthracyclines. Dexrazoxane is available under brand names, including Totect and Zinecard.
Dexrazoxane Structure and Mechanism of Action 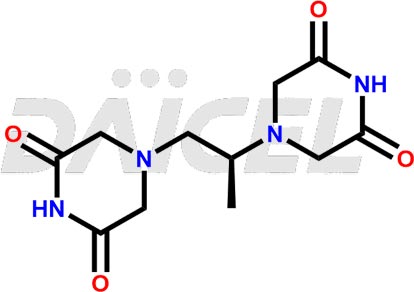
The chemical name of Dexrazoxane is 4,4′-[(1S)-1-Methyl-1,2-ethanediyl]bis[2,6-piperazinedione]. Its chemical formula is C11H16N4O4, and its molecular weight is approximately 268.27 g/mol.
Dexrazoxane readily penetrates cell membranes and is a cyclic derivative of EDTA.
Dexrazoxane Impurities and Synthesis
During the synthesis1 of Dexrazoxane, impurities generate as byproducts. They may originate from starting materials, reagents, or reaction intermediates used in manufacturing. It is necessary to identify and characterize these impurities to ensure the purity and safety of the final product. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) help in impurity profiling and quantification. Manufacturers of Dexrazoxane closely monitor impurities to meet regulatory standards and implement purification methods to minimize their presence, ensuring the highest quality of the drug for therapeutic use.
Daicel Pharma offers a Certificate of Analysis (CoA) for Dexrazoxane impurity standards, such as Dexrazoxane Impurity-A, Dexrazoxane Impurity-C, and Dexrazoxane Impurity-D, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Dexrazoxane impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Creighton, Andrew M., 3,5-dioxopiperazine derivatives, National Research Development Corp., GB1374979A, November 20, 1974
- Rosing, H.; van Gijn, R.; ten Bokkel Huinink, W. W.; Beijnen, J. H., High performance liquid chromatographic analysis of the cardioprotective agent dexrazoxane in human plasma and urine, Journal of Liquid Chromatography & Related Technologies, Volume: 20, Issue: 4, Pages: 583-601, 1997
Frequently Asked Questions
What are the common impurities found in Dexrazoxane?
The common impurities in Dexrazoxane may include related substances, degradation products, residual solvents, and other process-related contaminants. They vary depending on the synthetic route and manufacturing process.
How are Dexrazoxane impurities removed during the purification of the drug?
Impurities in Dexrazoxane are removed during purification steps such as crystallization, filtration, chromatography, and other separation techniques. These methods help isolate the desired product while removing impurities in the reaction mixture.
Which solvent helps in the analysis of Dexrazoxane impurities?
Water is a solvent used in analyzing many impurities in Dexrazoxane.
What are the temperature conditions required to store Dexrazoxane impurities?
Dexrazoxane impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.