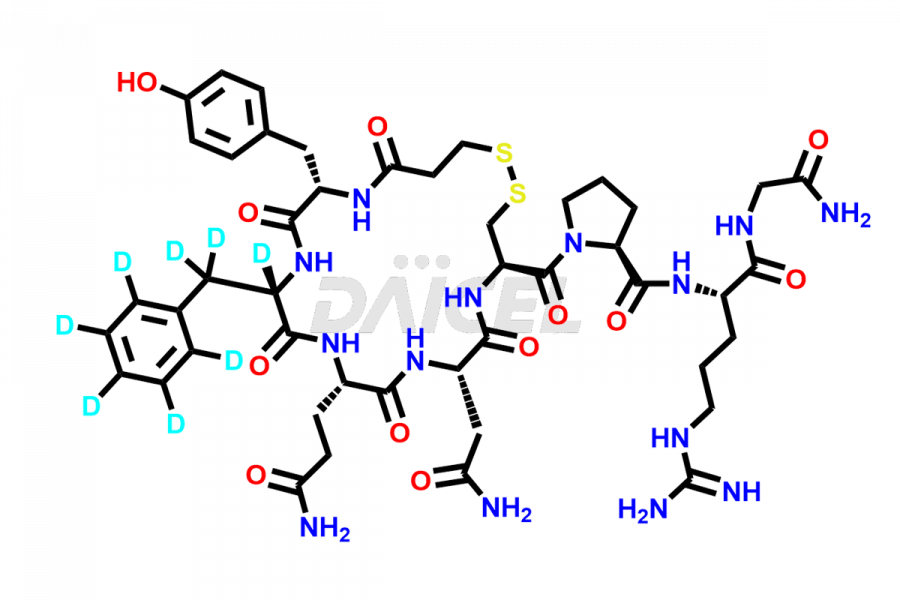
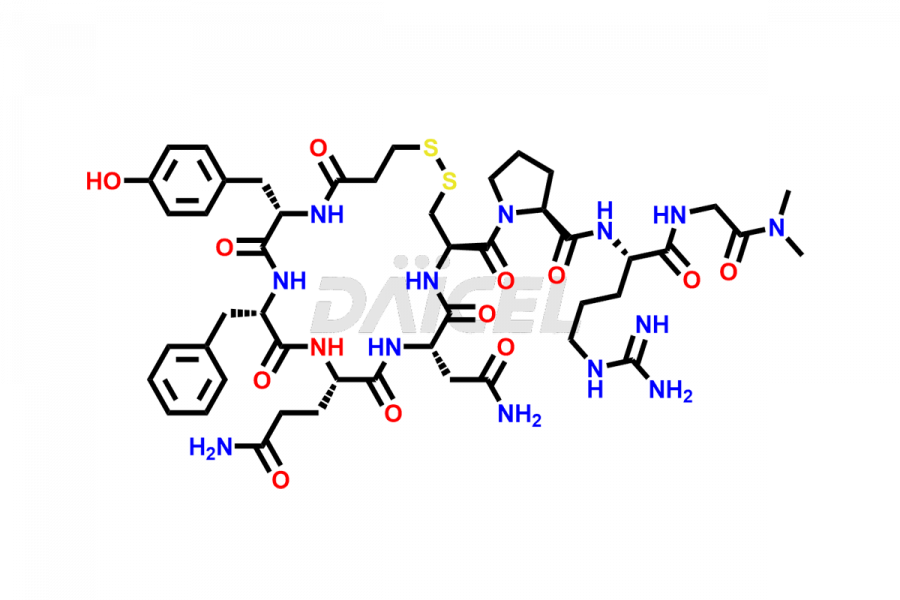
Desmopressin
General Information
Desmopressin Impurities and Desmopressin
Daicel Pharma synthesizes high-quality Desmopressin impurity, N, N’-dimethyl-Gly-Desmopressin, which helps in the quality, stability, and biological safety analysis of Desmopressin. We also offer custom synthesis of Desmopressin impurities and supply worldwide.
Desmopressin [CAS: 16679-58-6], a peptide of nine amino acids, is a synthetic analog of the natural pituitary hormone, 8-arginine vasopressin, an antidiuretic hormone (ADH). It manages various medical conditions, such as von Willebrand disease, sickle cell disease, diabetes insipidus, and nocturnal enuresis.
Desmopressin: Use and Commercial Availability
Desmopressin is a drug with different forms of administration and indications. When administered intranasally, it is for adults with nocturia due to nocturnal polyuria and managing central cranial diabetes insipidus or temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. When administered parenterally, it is for maintaining hemostasis during surgical procedures and postoperatively in patients with hemophilia A or mild to moderate classic von Willebrand’s disease (Type I).
The availability of Desmopressin varies depending on the country and the type of medical treatment. The common brand names under which Desmopressin is available are DDAVP, DesmoMelt, Desmotabs, Noctiva, Minirin, Octim, and Stimate.
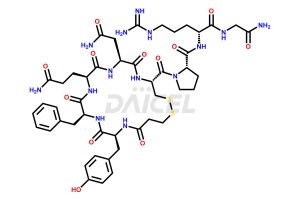
Desmopressin Structure and Mechanism of Action
The chemical formula of Desmopressin is C46H64N14O12S2, and its molecular weight is 1069.22 g/mol.
On binding of Desmopressin to V2 receptors in the basolateral membrane of the distal tubule cells and collecting ducts of the nephron adenylyl cyclase is stimulated. The resulting intracellular cascades in the collecting duct lead to an increased insertion rate of water channels, called aquaporins, into the lumenal membrane and enhance the membrane permeability to water1.
Desmopressin Impurities and Synthesis
Desmopressin is a synthetic peptide hormone analog, synthesized2 through multiple steps, including solid-phase peptide synthesis and chromatography purification. During the manufacturing process, there is a potential for impurities to arise, such as truncated or modified peptide chains, residual solvents, and heavy metals. Strict quality control measures are taken to ensure the purity and quality of Desmopressin, including testing for impurities using HPLC and liquid chromatography-mass spectrometry (LC-MS). The synthesis and quality control of Desmopressin is critical to ensure the purity and efficacy of the medication.
At Daicel, we provide a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Desmopressin impurity standard such as N, N’-dimethyl-Gly-Desmopressin, along with complete characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Upon request, we provide 13C-DEPT and CHN. Also, we provide a complete characterization report upon delivery. Daicel offers Desmopressin D8, which is a deuterium-labeled standard of Desmopressin in bioanalytical research and BA/BE studies with isotope data in CoA.
References
FAQ's
References
- (https://pubchem.ncbi.nlm.nih.gov/compound/5311065)
- Milan Zaoral, Ivan Vavra, Alena Machova, and Frantisek Sorm, Ceskoslovenska Akademie Ved., Prague, Czechoslovakia, “1-DEAMINO-8-D-ARGININE VASOPRESSIN”, US 3,497,491, Feb 24, 1970
- Lindeberg, “Separation of vasopressin analogs by reversed-phase high-performance liquid chromatography” Journal of Chromatography, Volume: 193, Issue: 3, Pages: 427-31, 1980
Frequently Asked Questions
How are Desmopressin impurities synthesized?
The synthesis of Desmopressin impurities involves the chemical synthesis of the peptide chain using solid-phase peptide synthesis (SPPS) techniques. The desired peptide sequence is built upon a solid support resin, and the crude product is then cleaved from the resin and purified using HPLC or other chromatography techniques. The purified peptide is subjected to various chemical modifications to generate the desired impurities. These modifications may include truncation of the peptide chain, acylation, oxidation, or other modifications. The final impurities are characterized using analytical techniques such as HPLC, and liquid chromatography-mass spectrometry (LC-MS).
How are Desmopressin impurities identified and quantified during the manufacturing process?
Desmopressin impurities are identified and quantified during the manufacturing process using techniques such as high-performance liquid chromatography3 (HPLC),and liquid chromatography-mass spectrometry (LC-MS). These techniques help to identify the type and quantity of impurities present in the sample.
What are the chromatography techniques used in the purification of Desmopressin impurities?
Chromatography is used to purify Desmopressin impurities to separate the desired product from other unwanted components in the mixture. Chromatography techniques, such as reversed-phase high-performance liquid chromatography (RP-HPLC), are commonly used for peptide purification and to ensure the purity and quality of the final product.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.