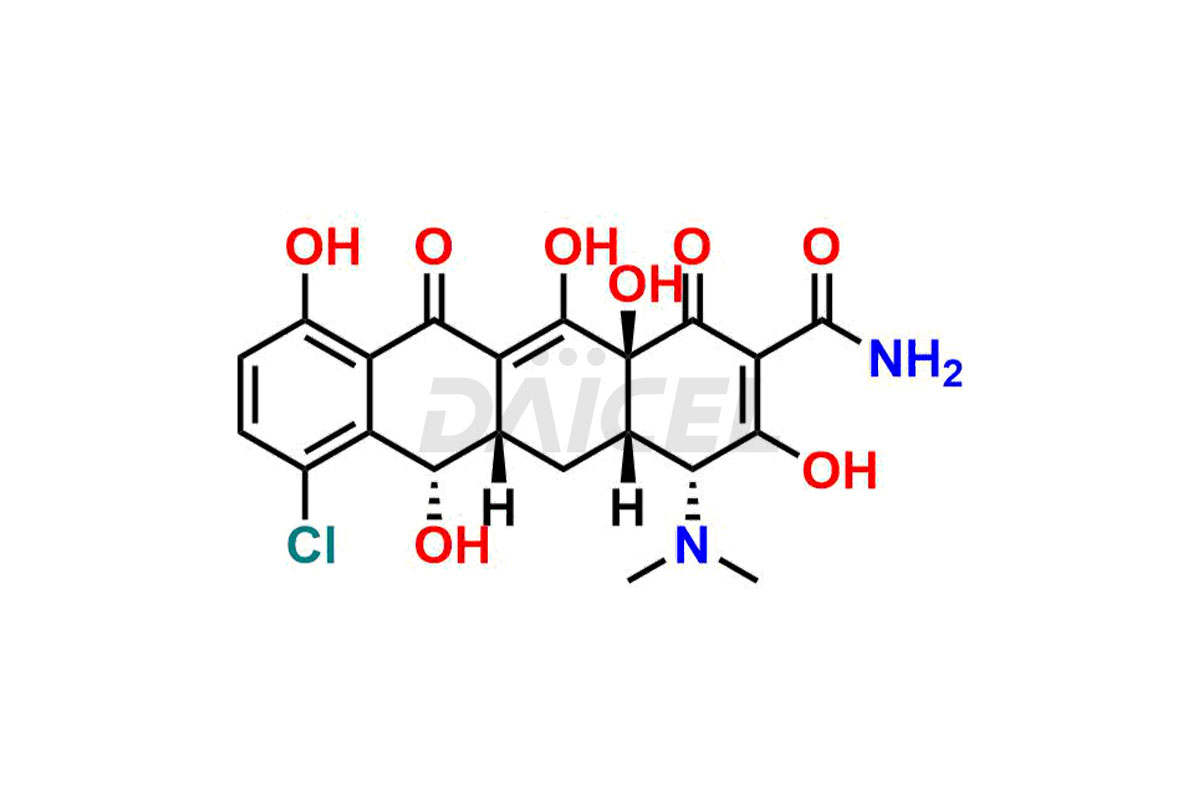
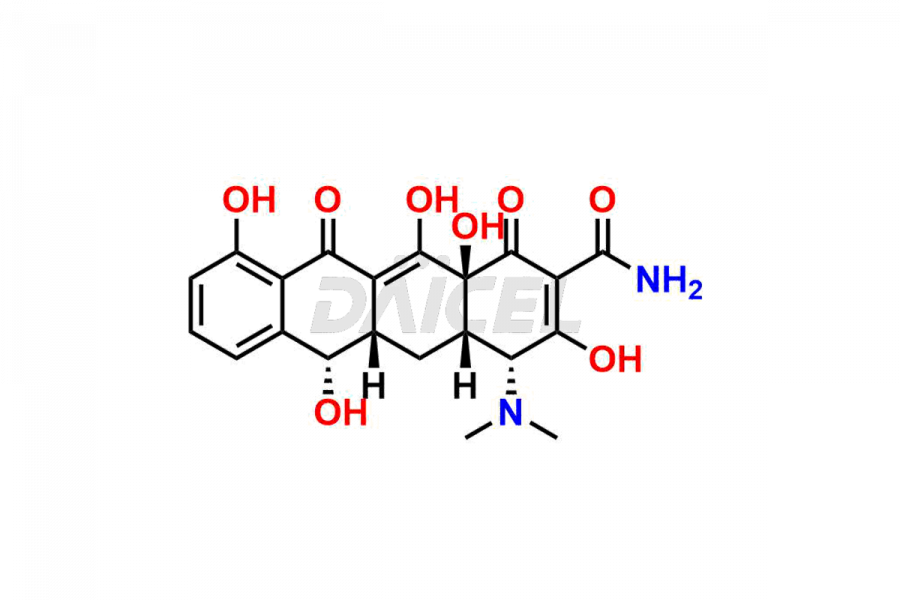
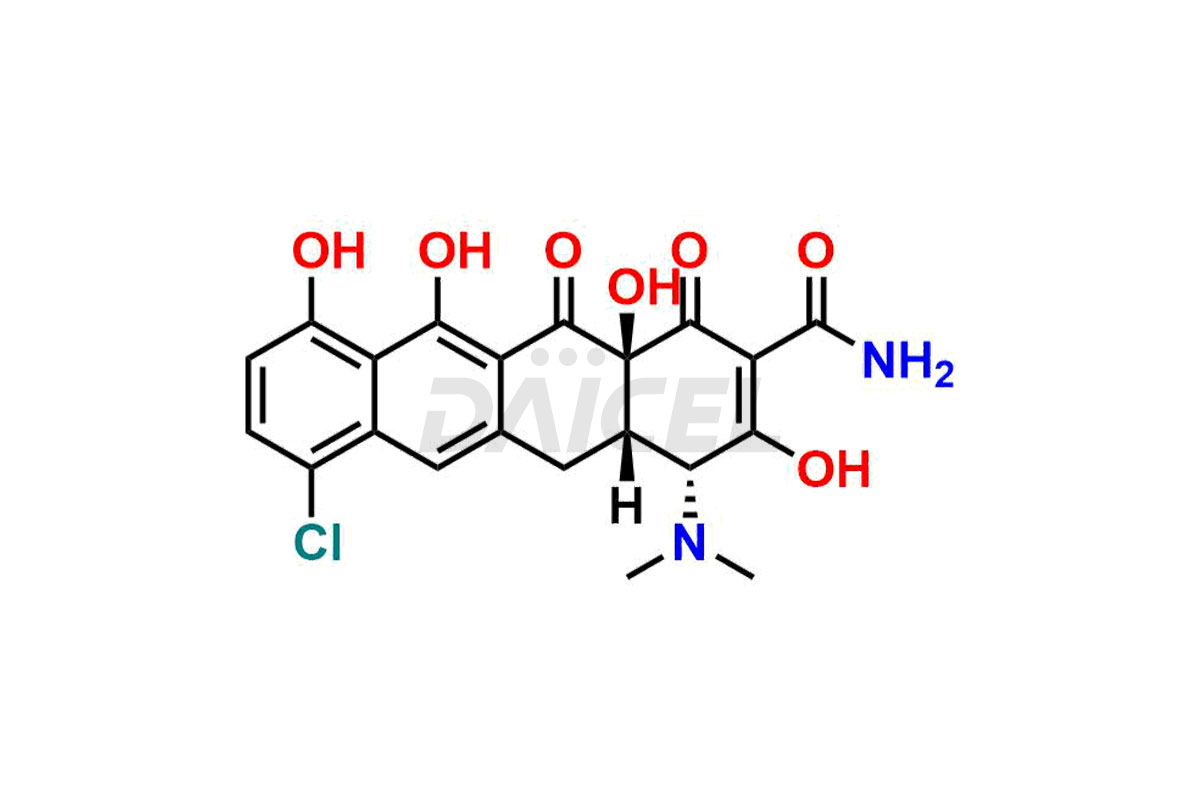
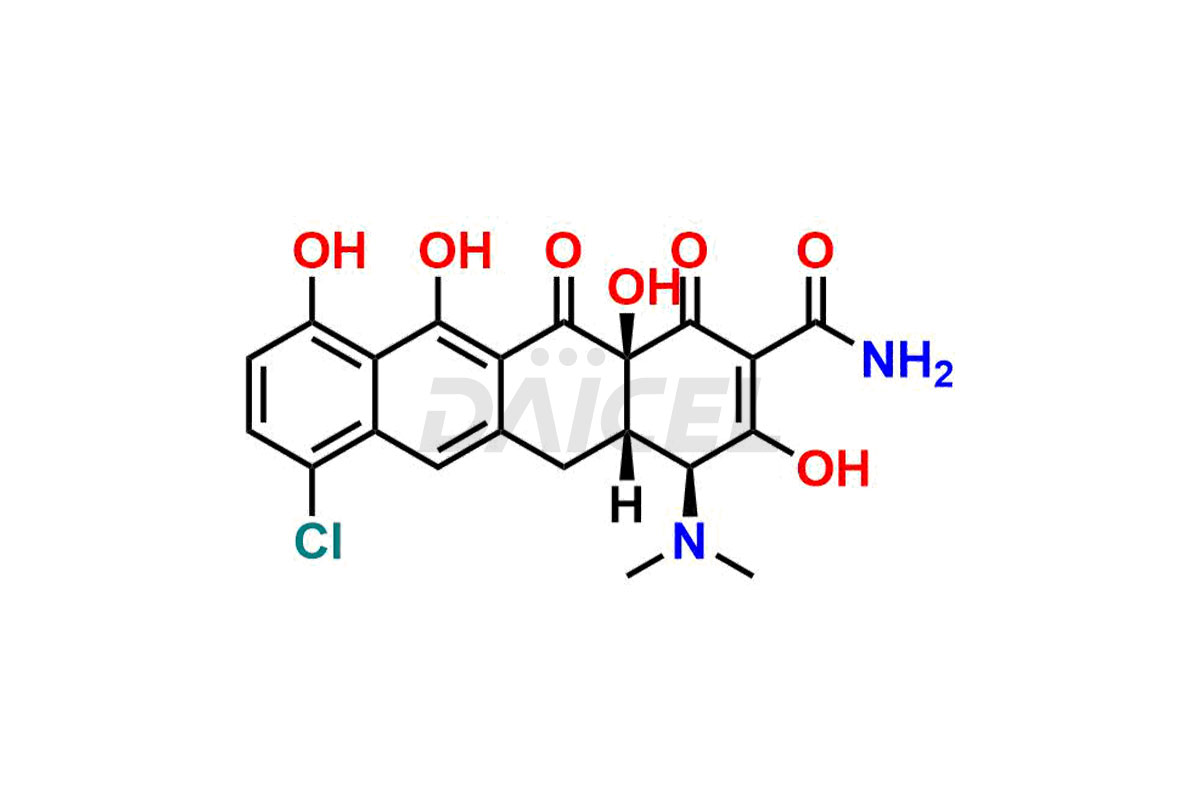
Demeclocycline
General Information
Demeclocycline Impurities and Demeclocycline
Daicel Pharma synthesizes Demeclocycline impurities of exceptional quality, such as 4-Epidemeclocycline, 4-Epidemethyltetracycline, Demeclocycline Impurity F, Demeclocycline Impurity G, and Demethyltetracycline Impurity-A. These impurities are crucial to assess the purity, reliability, and safety of Demeclocycline, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Demeclocycline impurities to meet clients’ demands for delivery worldwide.
Demeclocycline [CAS: 127-33-3], a semi-synthetic tetracycline derived from Streptococcus aureofaciens, is an antibiotic and arginine vasopressin inhibitor. It is a broad-spectrum naphthacene antibiotic. Compared to tetracycline, it has a slower excretion rate, allowing it to maintain blood levels for longer durations. Demeclocycline treats hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH).
Demeclocycline: Use and Commercial Availability
Demeclocycline, available under the tradename Declomycin, is a tetracycline antibiotic that exhibits broad-spectrum activity against gram-positive and gram-negative bacteria. It targets the underlying pathophysiology of SIADH.
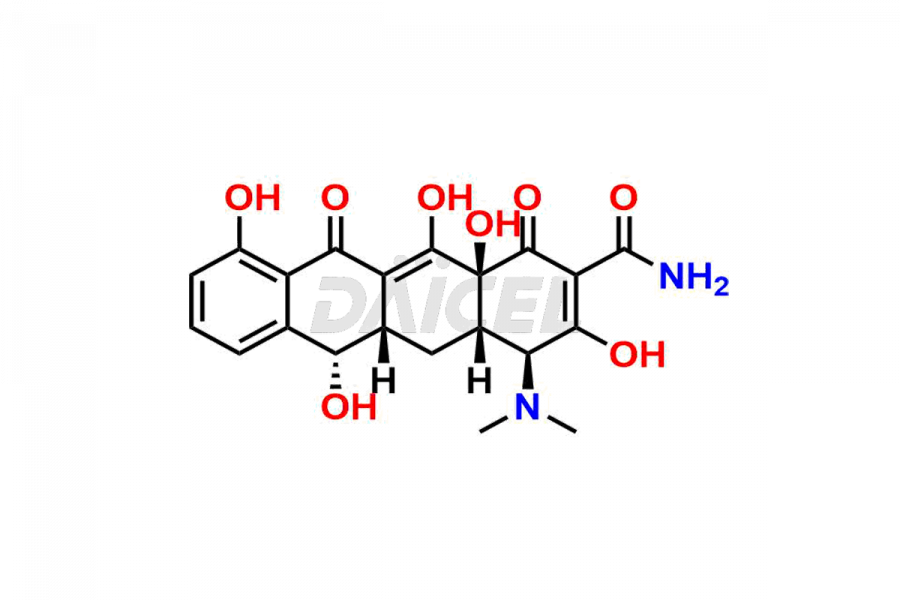
Demeclocycline Structure and Mechanism of Action 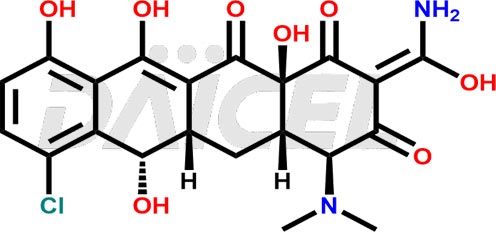
The chemical name of Demeclocycline is (4S,4aS,5aS,6S,12aS)-7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide. Its chemical formula is C21H21ClN2O8, and its molecular weight is approximately 464.9 g/mol.
The mechanism of action of Demeclocycline is not known.
Demeclocycline Impurities and Synthesis
Impurities in Demeclocycline can form through degradation of the active pharmaceutical ingredient, interaction with excipients, or contaminants in raw materials. They can affect the quality and efficacy of the drug product. Analytical methods such as chromatography and spectroscopy help analyze and identify Demeclocycline impurities. Their control measures involve establishing impurity level limits, following good manufacturing practices, and regular testing during the manufacturing process. Rigorous quality control and monitoring ensure that Demeclocycline batches meet regulatory requirements and maintain high levels of purity, ensuring the effectiveness and safety of the medication.
Daicel Pharma offers a Certificate of Analysis (CoA) for Demeclocycline impurity standards, such as 4-Epidemeclocycline, 4-Epidemethyltetracycline, Demeclocycline Impurity F, Demeclocycline Impurity G, and Demethyltetracycline Impurity-A, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Demeclocycline impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Naidong, Weng; Roets, E.; Hoogmartens, J., Quantitative analysis of demeclocycline by high-performance liquid chromatography, Journal of Pharmaceutical and Biomedical Analysis, Volume: 7, Issue: 12, Pages: 1691-703, 1989
- Li, Y. M.; Van Schepdael, A.; Roets, E.; Hoogmartens, J., Analysis of demeclocycline by capillary electrophoresis, Journal of Chromatography A, Volume: 740, Issue: 1, Pages: 119-123, 1996
Frequently Asked Questions
What measures help prevent the formation of impurities during Demeclocycline synthesis?
Preventing the formation of impurities during Demeclocycline synthesis involves optimizing reaction conditions, using pure starting materials and purification techniques, and conducting in-process controls to monitor impurity formation.
How are Demeclocycline impurities controlled during the storage and stability testing?
Controlling impurities during storage and stability testing of Demeclocycline involves monitoring the degradation and their formation over time. Stability studies under various storage conditions assess the drug's degradation profile and ensure that impurity levels remain within acceptable limits.
Which solvent helps in the analysis of Demeclocycline impurities?
DMSO or Methanol are the solvents used in analyzing many impurities in Demeclocycline.
What are the temperature conditions required to store Demeclocycline impurities?
Demeclocycline impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.