Decitabine
General Information
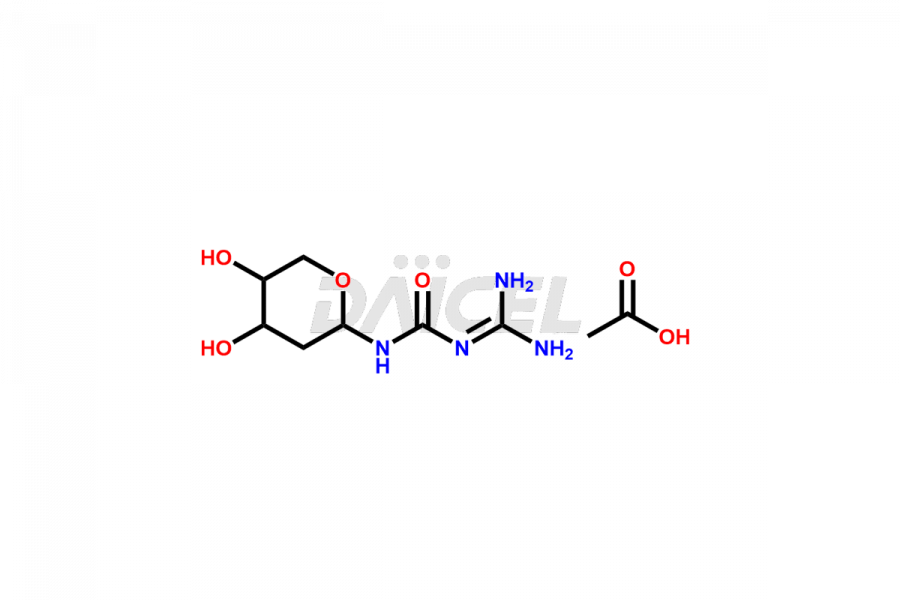
Decitabine Impurities and Decitabine
Daicel Pharma synthesizes Decitabine impurities of exceptional quality, such as Decitabine Hydrolyte Acetate and Decitabine Isomer-3. These impurities are crucial to assess the purity, reliability, and safety of Decitabine, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Decitabine impurities to meet clients’ demands for global delivery.
Decitabine [CAS: 2353-33-5], a cytosine analog, is an antineoplastic agent for treating myelodysplastic syndromes and acute myeloid leukemia. It is an azacitidine derivative and limits neoplasm drug resistance and metastasis.
Decitabine: Use and Commercial Availability
Decitabine, available under the brand name Dacogen, treats myelodysplastic syndromes (MDS) across all French-American-British subtypes, including refractory anemia, refractory anemia with excess blasts, chronic myelomonocytic leukemia, etc.
Decitabine Structure and Mechanism of Action 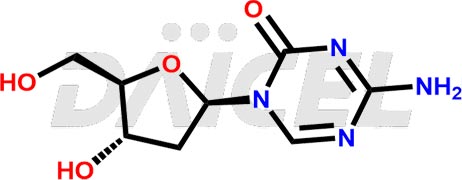
The chemical name of Decitabine is 4-Amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one. Its chemical formula is C8H12N4O4, and its molecular weight is approximately 228.21 g/mol.
Decitabine inhibits DNA methyltransferase, leading to DNA hypomethylation and apoptosis.
Decitabine Impurities and Synthesis
Impurities in Decitabine can arise from various sources, including degradation of the active pharmaceutical ingredient, reaction with excipients, or contaminants in raw materials. They can affect the drug’s safety, efficacy, and stability. Therefore, rigorous analysis and control of impurities are crucial. Analytical methods such as chromatography and spectroscopy help detect and quantify impurities in Decitabine. The control strategies involve setting impurity limits, implementing good manufacturing practices, and ensuring proper storage and handling of raw materials. Regular testing throughout the manufacturing process1 and stability studies helps monitor and control impurity levels, ensuring the quality of Decitabine.
Daicel Pharma offers a Certificate of Analysis (CoA) for Decitabine impurity standards, such as Decitabine Hydrolytes Acetate and Decitabine Isomer-3, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Decitabine impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Frantisek Sorm, Alois Piskal, Process for the manufacture of 1-glycosyl-5-azacytosines, Ceskoslovenska Akademie Ved., US3350388A, October 31, 1967
- Lin, Kun Tsan; Momparler, Richard L.; Rivard, Georges E., Sample preparation for the determination of 5-aza-2'-deoxycytidine in plasma by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 345, Issue: 1, Pages: 162-7, 1985
Frequently Asked Questions
How are impurity profiles established for Decitabine?
Impurity profiles for Decitabine are established through extensive testing of batches produced under different conditions, including stress testing, forced degradation studies, and long-term stability studies.
Are there specific quality control measures in place for Decitabine impurities?
The specific quality control measures involve setting impurity limits, regular testing during manufacturing, and ensuring compliance with regulatory guidelines.
Which solvent helps in the analysis of Decitabine impurities?
Cold water is the solvent used in analyzing many impurities in Decitabine.
What are the temperature conditions required to store Decitabine impurities?
Decitabine impurities are stored at a controlled room temperature between -20°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.