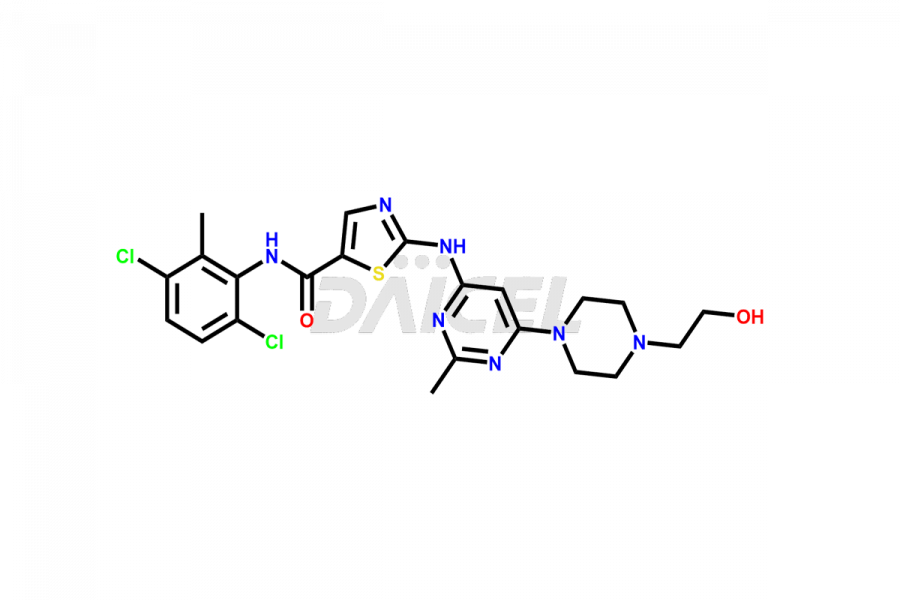
Dasatinib
References
- Das, Jagabandhu; Padmanabha, Ramesh; Chen, Ping; Norris, Derek J.; Doweyko, Arthur M. P.; Barrish, Joel C.; Wityak, John, Cyclic protein tyrosine kinase inhibitors Bristol-Myers Squibb Co., United States, US6979694B2, Dec 27, 2005
- Mhaske, D. V.; Dhaneshwar, S. R., Stability indicating HPTLC and LC determination of dasatinib in pharmaceutical dosage form, Chromatographia, Volume: 66, Issue: 1/2, Pages: 95-102, 2007
Frequently Asked Questions
What are the regulatory requirements for impurity levels in Dasatinib?
Regulatory authorities, such as the United States Pharmacopeia (USP) and the International Conference on Harmonization (ICH), provide guidelines and limits for impurity levels in pharmaceutical products, including Dasatinib.
How are Dasatinib impurities identified and characterized in the drug?
Impurities in Dasatinib are identified and characterized using analytical techniques such as high-resolution mass spectrometry (HRMS) and comparison with reference standards.
What steps help minimize the formation of Dasatinib impurities during the drug synthesis?
The steps to minimize impurity formation during Dasatinib synthesis are optimizing reaction conditions, controlling temperature and pH, using appropriate catalysts and reagents, and implementing effective purification techniques.
What are the temperature conditions required to store Dasatinib impurities?
Dasatinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.