LOAD MORE
You're viewed 9 of 31 products
Daicel Pharma synthesizes Darunavir impurities of exceptional quality, such as (3R,3aR,6aS)-Darunavir isomer, (3S,3aR,6aS)-Darunavir isomer, Amprenevir Oxazolone, BIS THF HNS Derivative-I, and more. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Darunavir. Besides, Daicel Pharma provides custom synthesis of Darunavir impurities to meet clients’ demands for delivery worldwide.
Darunavir [CAS: 206361-99-1] is an essential antiretroviral medication classified as a protease inhibitor, used for both therapy and prevention of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS). It helps manage HIV/AIDS by suppressing viral replication and reducing the progression of the disease.
Darunavir, available under the brand name, Prezista, is an antiretroviral medication for treating human immunodeficiency virus (HIV-1) infection. Its administered in combination with other antiretroviral drugs, either low-dose ritonavir or cobicistat, depending on the patient’s age and weight. It is an essential component of antiretroviral therapy, particularly for ART-experienced patients, contributing to effective HIV management and improved patient outcomes.
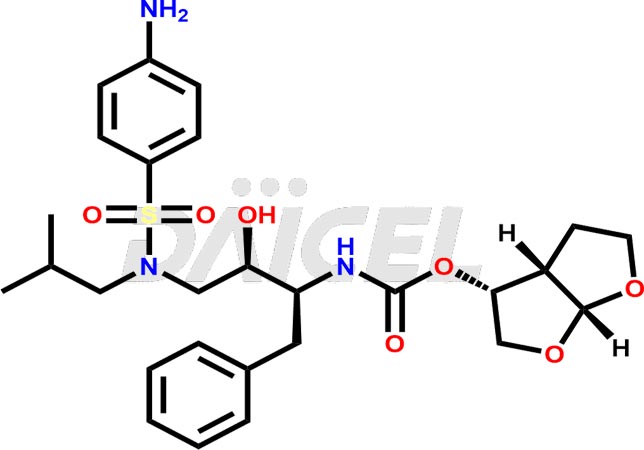
The chemical name of Darunavir is (3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl N-[(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamate. Its chemical formula is C27H37N3O7S, and its molecular weight is approximately 547.7 g/mol.
Darunavir blocks the protease enzyme that reproduces HIV. The virus does not reproduce normally, thus slowing down its multiplication in the body.
During manufacturing1, storage, or degradation of Darunavir, impurities form due to various processes. These impurities can arise from raw materials, reaction intermediates, or degradation of the drug substance. Analytical techniques such as HPLC, LC, and MS help analyze and quantify these impurities. It is crucial to control these impurities to ensure Darunavir’s quality, safety, and efficacy of Darunavir. Stringent control measures involve setting impurity specifications and implementing robust manufacturing processes, which are necessary.
Daicel Pharma offers a Certificate of Analysis (CoA) for Darunavir impurity standards, such as (3R,3aR,6aS)-Darunavir isomer, (3S,3aR,6aS)-Darunavir isomer, Amprenevir Oxazolone, BIS THF HNS Derivative-I, and more, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Darunavir impurities or degradation products. A complete characterization report accompanies every delivery.
Yes, stability studies are conducted to monitor impurity levels in Darunavir over its shelf life, ensuring the levels of impurities remain within acceptable limits.
Impurity control is an ongoing process that starts from the early development stages and continues throughout the entire lifecycle of Darunavir, including post-marketing surveillance.
Acetonitrile is a solvent used in analyzing many impurities in Darunavir.
Darunavir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.