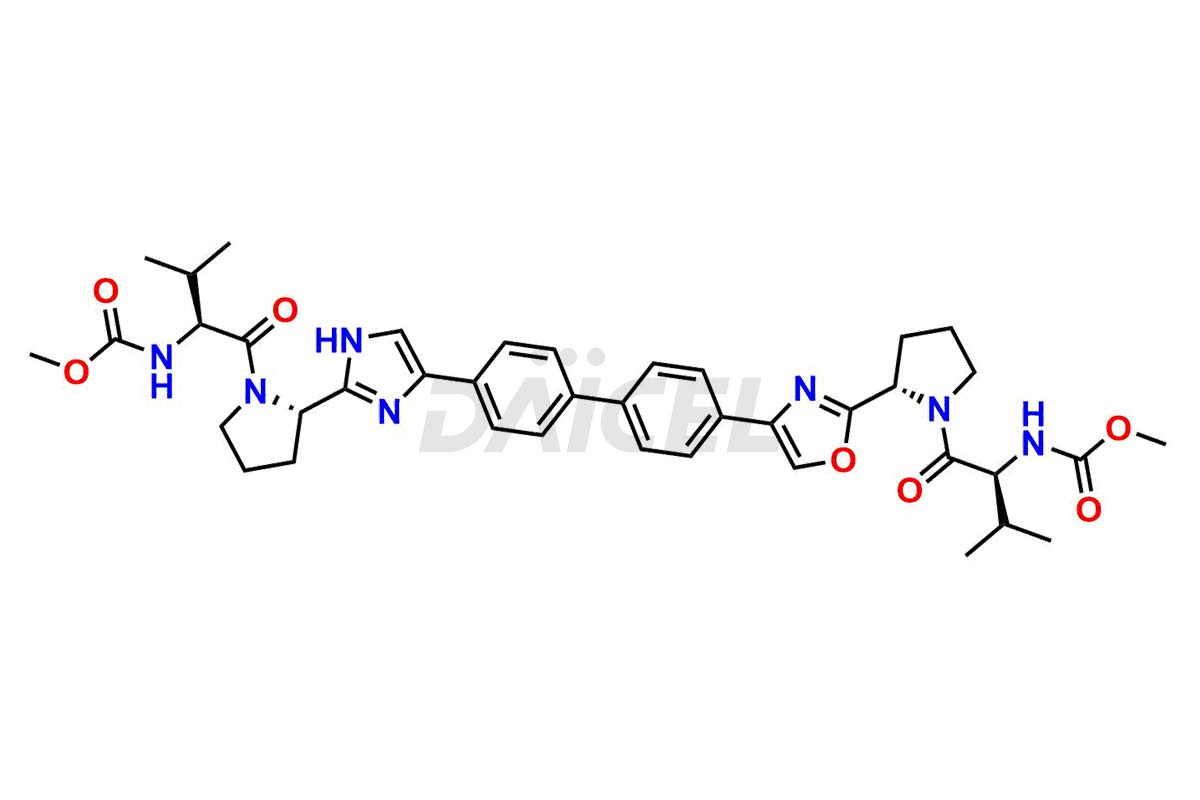
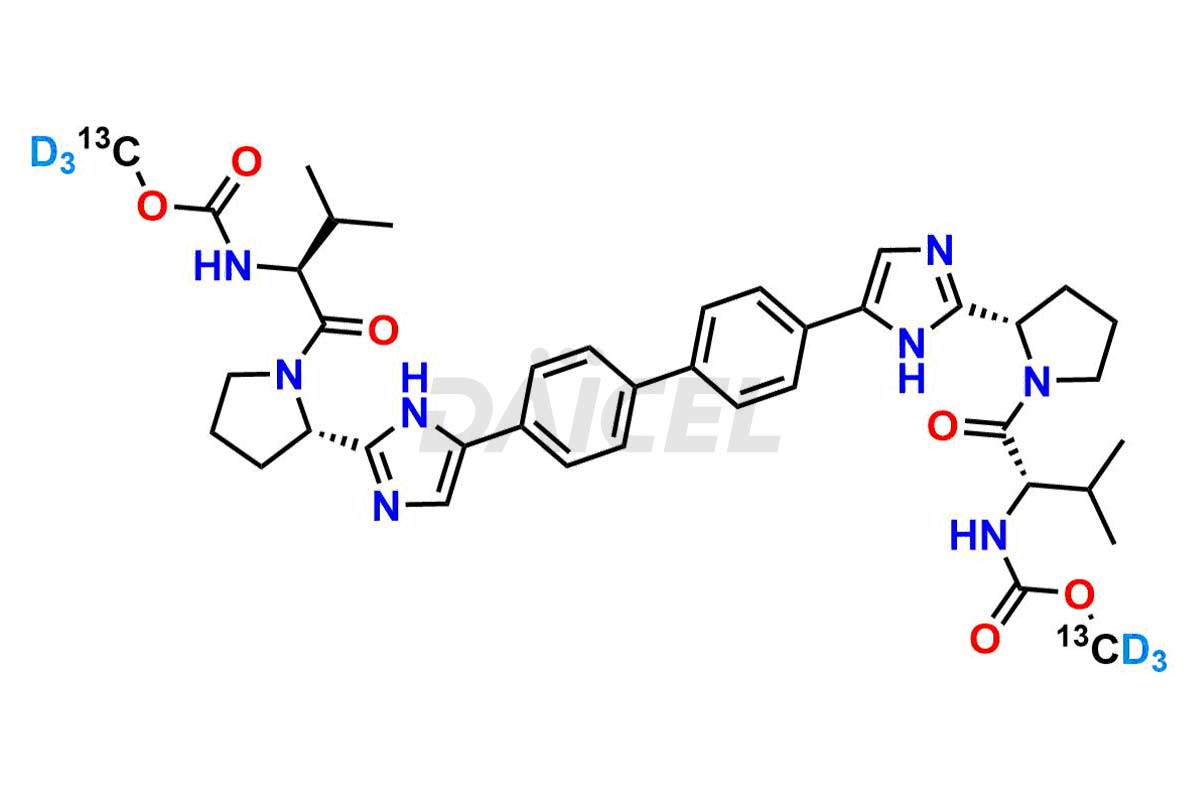
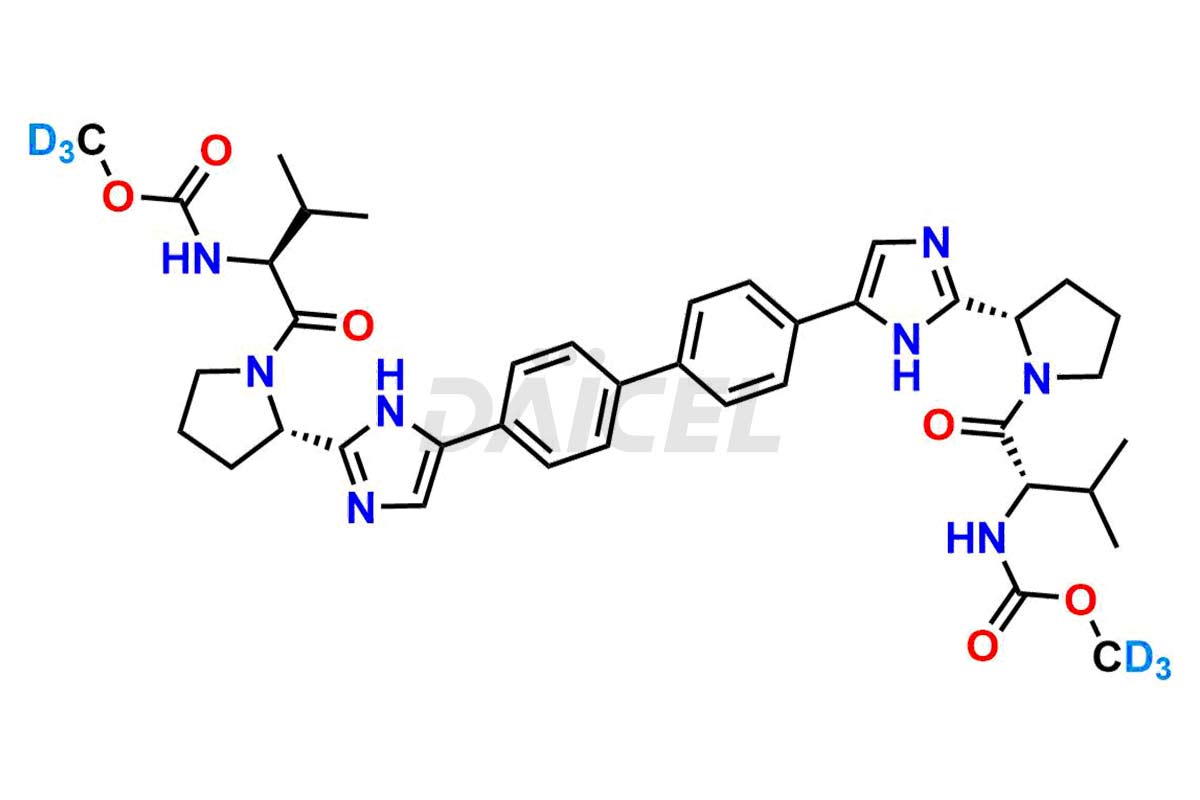
Daclatasvir
General Information
Daclatasvir Impurities and Daclatasvir
Daicel Pharma synthesizes Daclatasvir impurity of exceptional quality, Daclatasvir Impurity G. This impurity is crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Daclatasvir. Besides, Daicel Pharma provides custom synthesis of Daclatasvir impurities to meet clients’ demands for delivery worldwide.
Daclatasvir [CAS: 1009119-64-5] is an orally administered antiviral medication that specifically targets the nonstructural protein 5A (NS5A) region of the hepatitis C virus (HCV). It treats patients with chronic hepatitis C.
Daclatasvir: Use and Commercial Availability
Daclatasvir, available under the brand name Daklinza, in combination with sofosbuvir, treats chronic hepatitis C virus (HCV) genotype three infections. It targets the NS5A protein of HCV and prevents viral replication.
Daclatasvir Structure and Mechanism of Action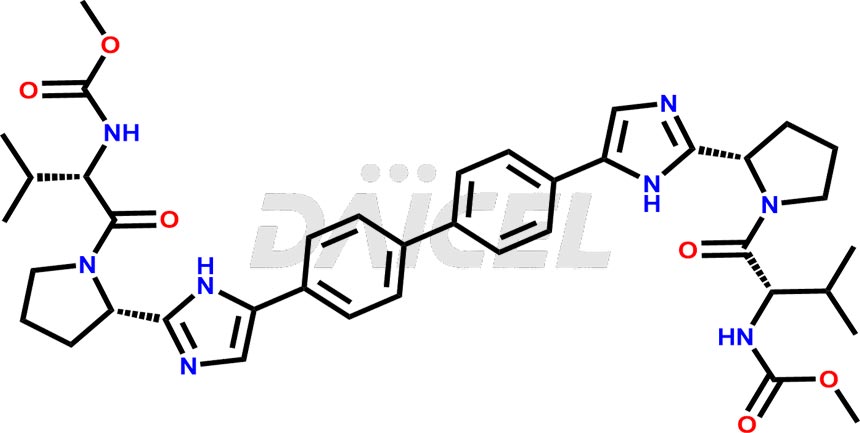
The chemical name of Daclatasvir is Carbamic acid, N, N′-[[1,1′-biphenyl]-4,4′-diylbis[1H-imidazole-5,2-diyl-(2S)-2,1-pyrrolidinediyl[(1S)-1-(1-methyl ethyl)-2-oxo-2,1-ethanediyl]]]bis-, C, C′-dimethyl ester. Its chemical formula is C40H50N8O6, and its molecular weight is approximately 738.9 g/mol.
Daclatasvir inhibits NS5A, a nonstructural protein encoded by Hepatitis C Virus. It binds to the N-terminus of NS5A and prevents replication of viral RNA and virion assembly.
Daclatasvir Impurities and Synthesis
Daclatasvir impurities form during the manufacturing1, storage, or degradation processes. Analytical techniques such as HPLC, LC, and MS help identify and quantify these impurities. It is crucial to control these impurities as they can affect the quality and safety of Daclatasvir. Strict implementation of controlling impurity specifications and robust manufacturing processes are necessary to ensure the purity and efficacy of the drug. Compliance with regulatory guidelines and continuous monitoring is essential to maintain the quality and effectiveness of Daclatasvir.
Daicel Pharma offers a Certificate of Analysis (CoA) for the Daclatasvir impurity standard, Daclatasvir Impurity G, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Daclatasvir impurities or degradation products and labeled compounds to assess the effectiveness of generic Daclatasvir. We also offer Daclatasvir-13C2D6 and Daclatasvir-D6, deuterium-labeled Daclatasvir compounds useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Bachand, Carol; Belema, Makonen; Deon, Daniel H.; Good, Andrew C.; Goodrich, Jason; James, Clint A.; Lavoie, Rico; Lopez, Omar D.; Martel, Alain; Meanwell, Nicholas A.; et al, Hepatitis C virus inhibitors, Bristol-Myers Squibb Company, United States, US8329159B2, December 11, 2012
- Jiang, Hao; Zeng, Jianing; Kandoussi, Hamza; Liu, Yifei; Wang, Xiaodong; Bifano, Marc; Cojocaru, Laura; Ryan, John; Arnold, Mark E., A sensitive and accurate liquid chromatography-tandem mass spectrometry method for quantitative determination of the novel hepatitis C NS5A inhibitor BMS-790052 (daclastasvir) in human plasma and urine, Journal of Chromatography A, Volume: 1245, Pages: 117-121, 2012
Frequently Asked Questions
How are raw materials tested for impurities in Daclatasvir production?
Rigorous quality control procedures, including testing and qualification of raw materials, are implemented to ensure their purity and suitability for Daclatasvir manufacturing.
Can Daclatasvir impurities impact its therapeutic efficacy?
Some impurities in Daclatasvir may affect its therapeutic efficacy by interfering with its mechanism of action or causing undesired effects in the body.
Which solvent helps in the analysis of Daclatasvir impurities?
Methanol or water are the solvents used in analyzing many impurities in Daclatasvir.
What are the temperature conditions required to store Daclatasvir impurities?
Daclatasvir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.