Dabigatran
General Information
Dabigatran Impurities and Dabigatran
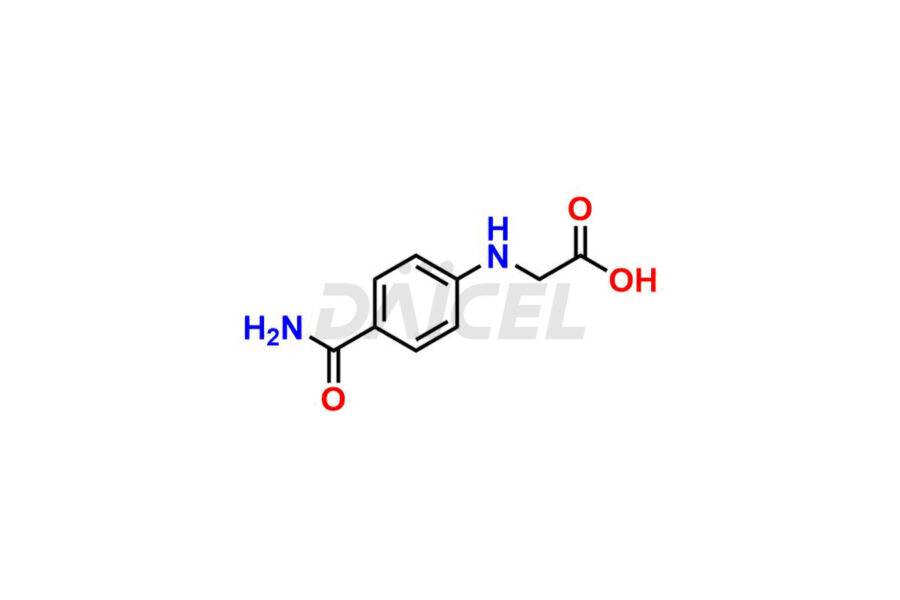
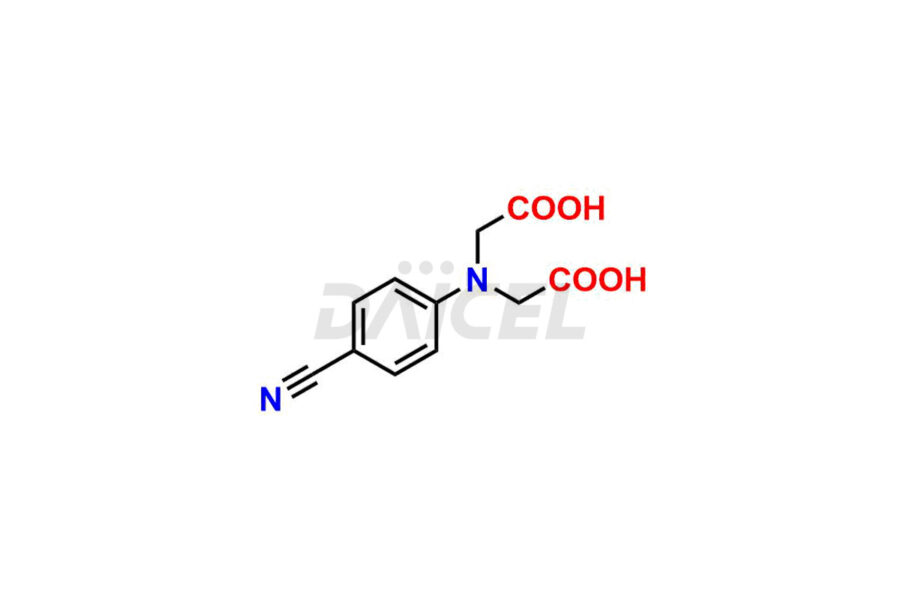
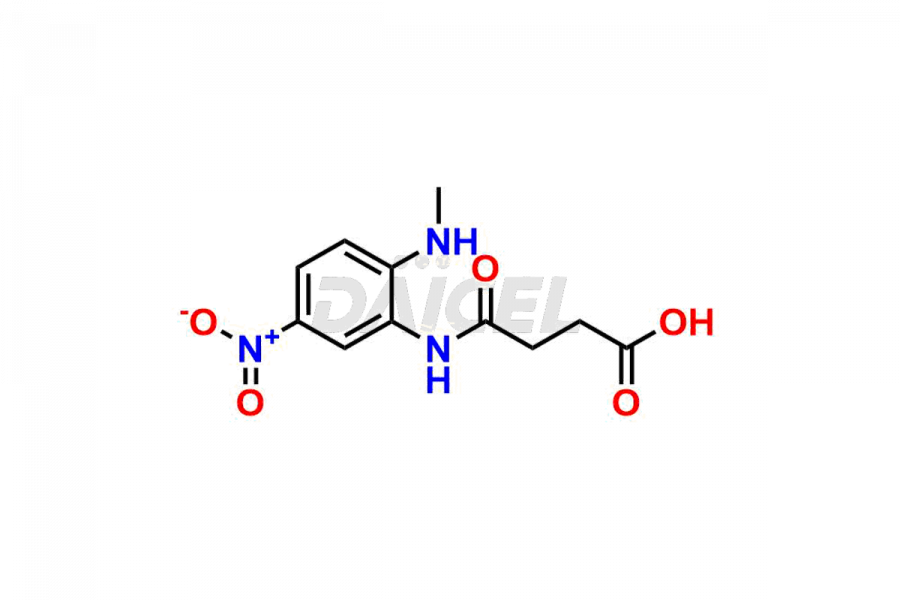
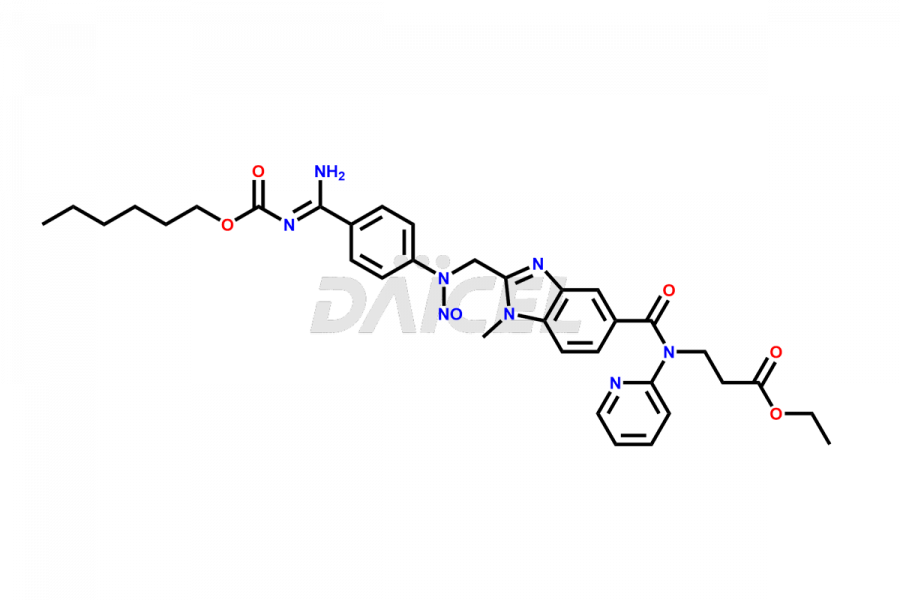
Daicel Pharma synthesizes Dabigatran impurities of exceptional quality, such as (4-carbamoylphenyl)glycine, 2,2′-((4-cyanophenyl)azanediyl)diacetic acid, 4-[2-(Methylamino)-5-nitro-anilino]-4-oxo-butanoic acid impurity, and N-Nitroso Dabigatran etexilate. These impurities are crucial to assess the purity, reliability, and safety of an active pharmaceutical ingredient, Dabigatran. Besides, Daicel Pharma provides custom synthesis of Dabigatran impurities to meet clients’ demands for delivery worldwide.
Dabigatran [CAS: 211914-51-1], the active metabolite of Dabigatran etexilate, is a direct thrombin inhibitor that acts as an anticoagulant. It prevents stroke and systemic embolism. Dabigatran provides effective prevention against stroke and venous embolism in patients with chronic atrial fibrillation.
Dabigatran: Use and Commercial Availability
Dabigatran is a potent small molecule that exerts its anticoagulant effects by directly binding to and inhibiting thrombin through ionic interactions at its active site. It effectively inhibits both free and clot-bound forms of thrombin rapidly and reversibly. Dabigatran etexilate, the prodrug form, undergoes first-pass metabolism to convert into the active derivative, Dabigatran, which has a relatively short half-life of approximately 40 minutes. The drug is available under the brand name Pradaxa.
Dabigatran Structure and Mechanism of Action 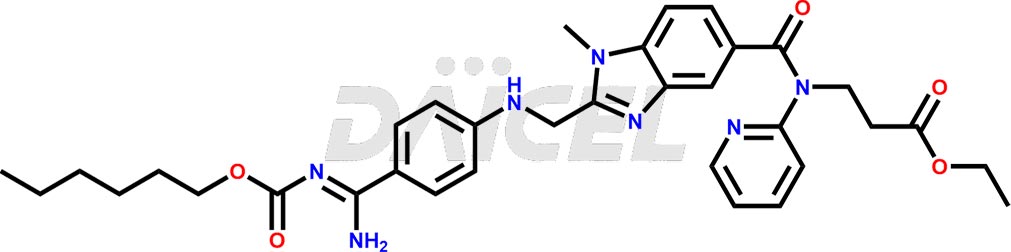
The chemical name of Dabigatran is N-[[2-[[[4-(Aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-β-alanine. Its chemical formula is C25H25N7O3, and its molecular weight is approximately 471.5 g/mol.
Dabigatran and its acyl glucuronides competitively inhibit thrombin. It inhibits the formation of free and clot-bound thrombin.
Dabigatran Impurities and Synthesis
During the productio1, storage, or degradation of Dabigatran, impurities may arise. These impurities form from various sources and may impact the quality and safety of the drug. Rigorous analysis using advanced techniques such as HPLC, LC, and MS is employed to identify and quantify these impurities. It is essential to implement stringent control measures to limit impurity levels and ensure the efficacy and safety of Dabigatran. Compliance with regulatory guidelines and continuous monitoring is necessary to maintain the purity and quality of the drug.
Daicel Pharma offers a Certificate of Analysis (CoA) for Dabigatran impurity standards, such as (4-carbamoyl phenyl)glycine, 2,2′-((4-cyanophenyl)azanediyl)diacetic acid, 4-[2-(Methylamino)-5-nitro-anilino]-4-oxo-butanoic acid impurity, and N-Nitroso Dabigatran etexilate, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Dabigatran impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Hauel, Norbert; Ries, Uwe; Priepke, Henning; Wienen, Wolfgang; Stassen, Jean Marie, Disubstituted Bicyclic Heterocycles, Their Production And Use As Medicaments, Boehringer Ingelheim Pharma K.-G., Germany, EP966454B1, May 7, 2003
- Delavenne, Xavier; Moracchini, Julie; Laporte, Silvy; Mismetti, Patrick; Basset, Thierry, UPLC MS/MS assay for routine quantification of dabigatran - a direct thrombin inhibitor - in human plasma, Journal of Pharmaceutical and Biomedical Analysis, Volume: 58, Pages: 152-156, 2012
Frequently Asked Questions
What are the potential risks associated with uncontrolled Dabigatran impurities?
Uncontrolled impurities in Dabigatran may pose risks such as reduced drug potency, increased toxicity, or harm to patients.
How are impurity limits determined for Dabigatran?
Impurity limits for Dabigatran are typically determined based on scientific and regulatory considerations and factors such as toxicological studies and stability data.
Can Dabigatran impurities impact its stability?
Some impurities in Dabigatran can affect its stability over time, leading to decreased shelf life or reduced potency.
What are the temperature conditions required to store Dabigatran impurities?
Dabigatran impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.