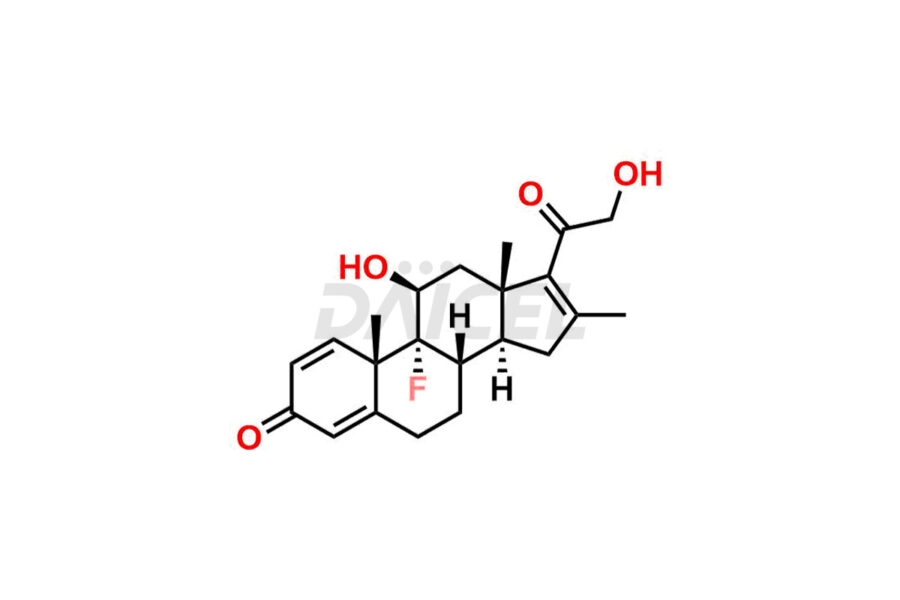
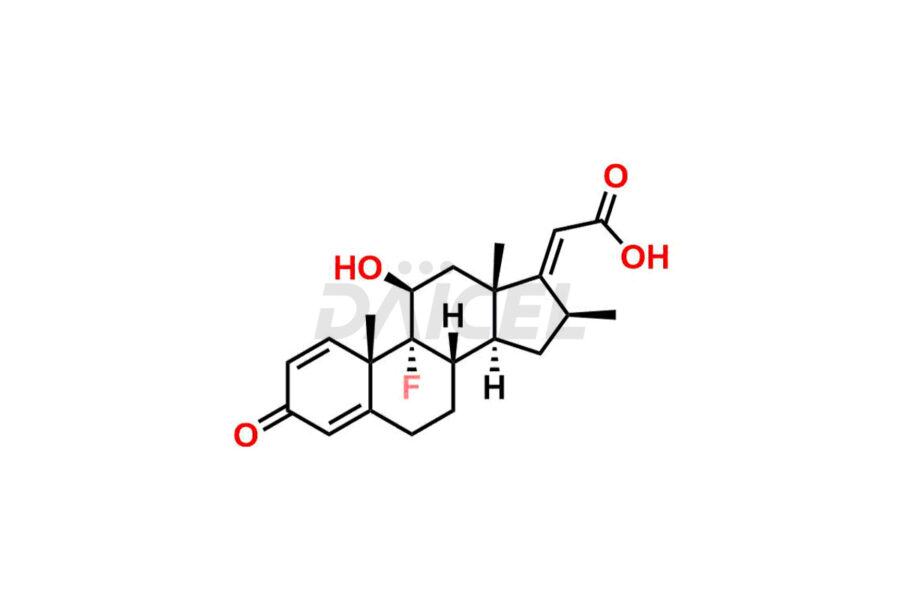
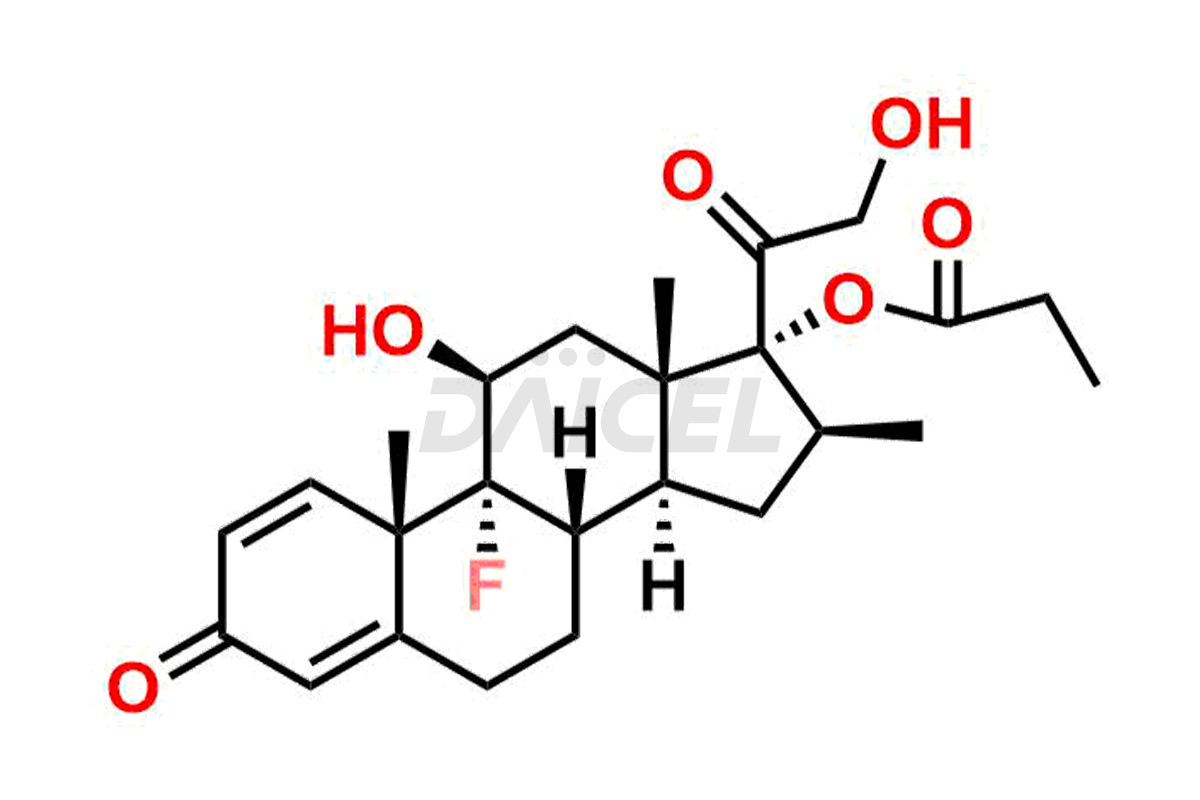
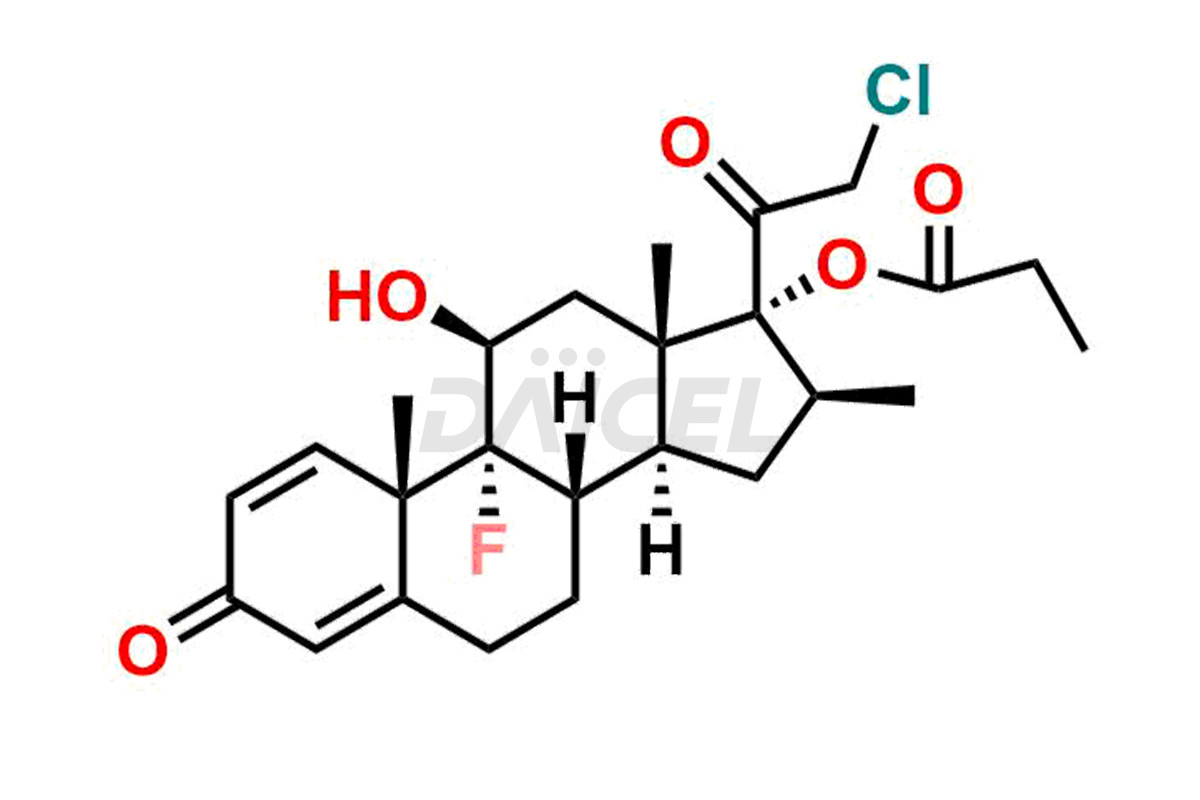
Clobetasol
General Information
Clobetasol Impurities and Clobetasol
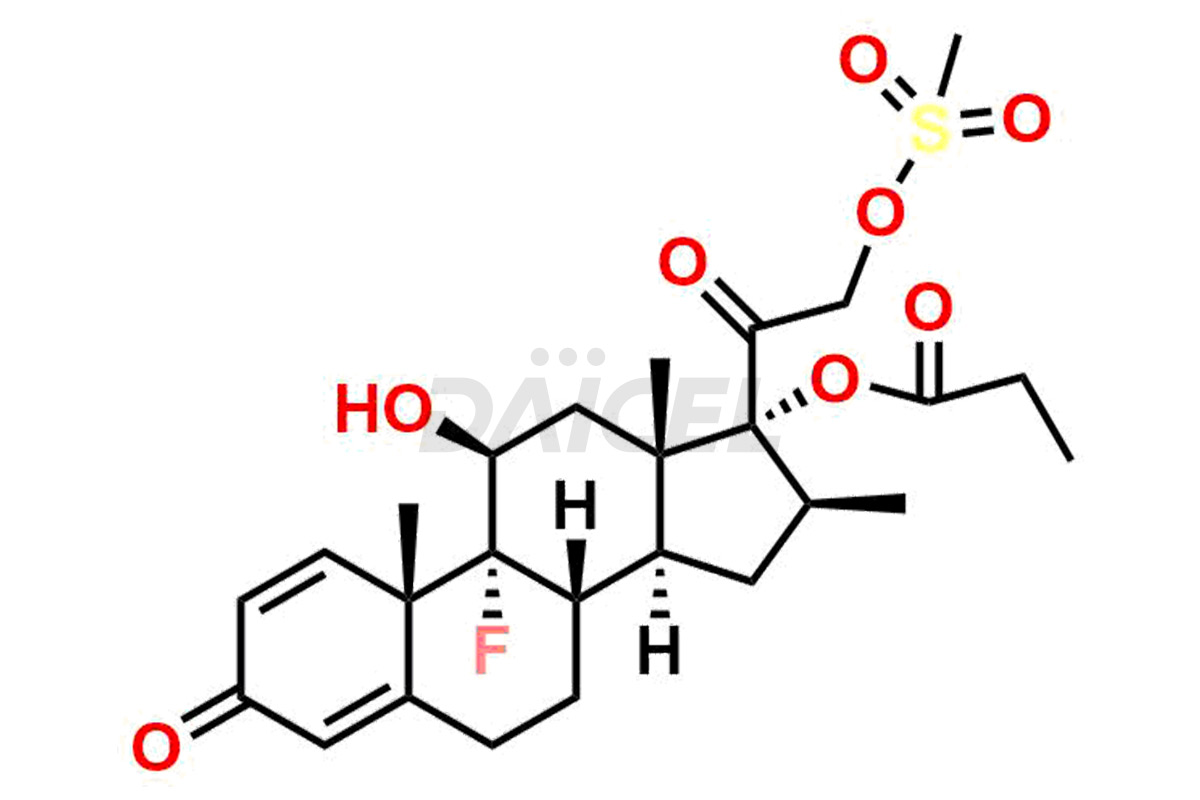
Daicel Pharma synthesizes high-quality Clobetasol impurities, Clobetasol 1,4,16-triene Impurity, Clobetasol Dipropionate 9-Fluoro Impurity, Clobetasol EP Impurity A, Clobetasol EP Impurity C, Clobetasol EP Impurity I, and Clobetasol propionate EP Impurity J, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Clobetasol. Moreover, Daicel Pharma offers custom synthesis of Clobetasol impurities and delivers them globally.
Topical corticosteroids, like Clobetasol [CAS: 25122-41-2], are synthetic steroids that treat inflammatory skin conditions and alleviate itching. These medicines have significantly improved the treatment of various inflammatory disorders.
Clobetasol: Use and Commercial Availability
Clobetasol is a potent topical corticosteroid used for the short-term treatment of severe and stubborn inflammatory skin conditions that do not respond to less potent corticosteroids. These conditions include eczemas, dermatitis, psoriasis, lichen planus, alopecia areata, and chronic discoid lupus erythematosus. It is available as a topical medication under different brand names like Clobetasol Propionate, Clobex, Cormax, Embeline, Impeklo, Impoyz, Olux, etc.
Clobetasol Structure and Mechanism of Action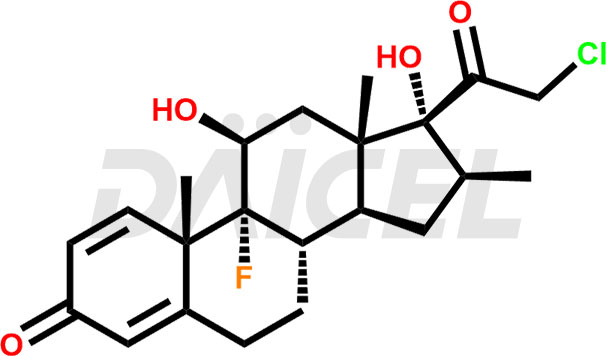
The chemical name of Clobetasol is (11β,16β)-21-Chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione. Its chemical formula is C22H28ClFO4, and its molecular weight is approximately 410.9 g/mol.
Clobetasol, like other corticosteroids, has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of action of Clobetasol is unknown.
Clobetasol Impurities and Synthesis
Clobetasol impurities1 arise due to factors like process-related impurities, degradation products, and storage and handling. Degradation impurities occur due to oxidation, isomerization, hydrolysis, and reduction reactions. The presence of these impurities can affect the stability and safety of Clobetasol preparations and the need to control them.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Clobetasol impurity standards, Clobetasol 1,4,16-triene Impurity, Clobetasol Dipropionate 9-Fluoro Impurity, Clobetasol EP Impurity A, Clobetasol EP Impurity C, Clobetasol EP Impurity I, and Clobetasol propionate EP Impurity J. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide a characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Clobetasol impurity or degradation product.
References
FAQ's
References
- NVVSS Narayana Murty Nagulakonda et al, Identification and quantitation of related substances of super potent steroid in its topical combination drug product with vitamin D3 analogue, Journal of Applied Pharmaceutical Science Vol. 9(08), pp 071-078, August, 2019,
- Nief Rahman Ahmed and Nawfal Sheet Mohamad, Spectrophotometric Determination Of Clobetasol Propionate In Pharmaceutical Preparations And Environmental Samples, World Journal Of Pharmacy And Pharmaceutical Sciences, Volume 7, Issue 10, 167173, 2018
Frequently Asked Questions
What are the related substances of Clobetasol?
The related substances of Clobetasol are chemical compounds structurally similar to Clobetasol and are present as impurities in Clobetasol preparations. These substances may include degradation products, synthesis impurities, and other compounds formed during the storage or handling of Clobetasol.
What methods can be used in the analysis of Clobetasol impurities?
Different analytical techniques, like HPLC or (RP-HPLC) Reversed-Phase High-Performance Liquid Chromatography, help identify and quantify Clobetasol impurities.
Which solvents help in the analysis of Clobetasol impurities?
Methanol or Acetonitrile are the solvents used for analyzing Clobetasol impurities.
What are the temperature conditions required to store Clobetasol impurities?
Clobetasol impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.