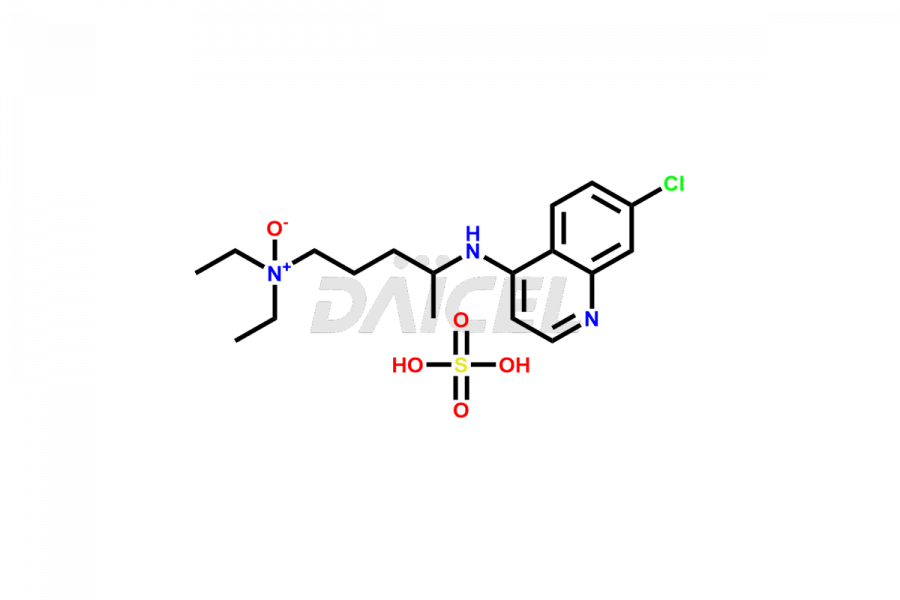
Chloroquine
General Information
Chloroquine Impurities and Chloroquine
Daicel Pharma synthesizes Chloroquine impurities of exceptional quality, such as Chloroquine Phosphate Impurity E and Chloroquine Phosphate impurity-1. These impurities are crucial to assess the purity, reliability, and safety of Chloroquine, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Chloroquine impurities to meet clients’ demands for delivery worldwide.
Chloroquine [CAS: 54-05-7] is a 4-aminoquinolone antimalarial agent. It is effective against the asexual erythrocytic forms of human plasmodia but inactive against the hepatic forms of this parasite. Apart from malaria treatment, Chloroquine also treats rheumatoid arthritis, various parasitic infections, and hepatic amebiasis.
Chloroquine: Use and Commercial Availability
Chloroquine is a medication that treats and prevents malaria caused by certain strains of Plasmodium, including P. falciparum, P. ovale, P. vivax, and P. malariae. Its use is in countries where Chloroquine-sensitive malaria is present, such as Mexico, parts of Central America, the Caribbean, East Asia, and some Middle Eastern countries. In addition to its antimalarial properties, Chloroquine treats extraintestinal amebiasis, a disease caused by a parasitic infection. Chloroquine also treats certain autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus. Chloroquine is available under brand names, including Aralen, Aralen Hydrochloride, and Chloroquine Phosphate.
Chloroquine Structure and Mechanism of Action 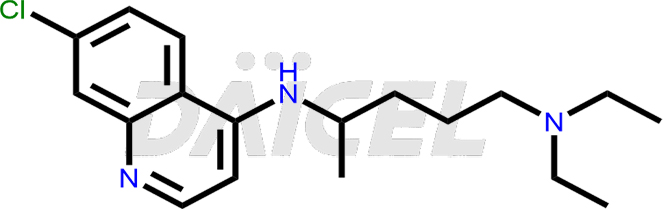
The chemical name of Chloroquine is 7-Chloro-4-[[4-(diethylamino)-1-methylbutyl]amino]quinoline. Its chemical formula is C18H26ClN3, and its molecular weight is approximately 319.9 g/mol.
Chloroquine inhibits certain enzymes due to its interaction with DNA. It prevents the polymerization of heme into hemozoin, causing toxic effects on the parasite.
Chloroquine Impurities and Synthesis
Impurities in Chloroquine develop during the synthesis1, storage, and drug degradation. They are classified as process-related, degradation-related, and external impurities. Process-related impurities result from incomplete reactions or unwanted by-products during the synthetic process. Degradation-related impurities form during storage or exposure to light, moisture, or temperature. External impurities occur during the handling and packaging of the drug substance. It is essential to control Chloroquine impurities as they can affect the drug’s purity, potency, safety, and efficacy. They may also lead to adverse effects and toxicities. The manufacturers must follow Good Manufacturing Practices (GMP) and use validated analytical methods to identify and quantify impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Chloroquine impurity standards, such as Chloroquine Phosphate Impurity E and Chloroquine Phosphate impurity-1, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we give additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Chloroquine impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Andersag, Hans; Breitner, Stefan; Jung, Heinrich, Quinoline Compound And Process Of Making The Same, Winthrop Chemical Co., US2233970A, March 4, 1941
- Bergqvist, Yngve; Frisk-Holmberg, Marianne, Sensitive method for the determination of chloroquine and its metabolite desethylchloroquine in human plasma and urine by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 221, Issue: 1, Pages: 119-27, 1980
Frequently Asked Questions
What is the impact of impurities on Chloroquine?
Impurities in Chloroquine can affect the drug's safety, efficacy, and stability. Some may be toxic or cause harm, while others may reduce the drug's potency or shelf-life.
How can the level of Chloroquine impurities be quantified?
The level of impurities in Chloroquine can be quantified using validated analytical methods, such as HPLC or LC-MS. Their amount is reported as a percentage of the drug substance.
How can Chloroquine impurities be minimized during manufacturing?
Chloroquine impurities may minimize during manufacturing by using high-quality raw materials, implementing GMPs, and using validated purification processes. Additionally, regular monitoring and testing can help to detect and control impurities.
What are the temperature conditions required to store Chloroquine impurities?
Chloroquine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.