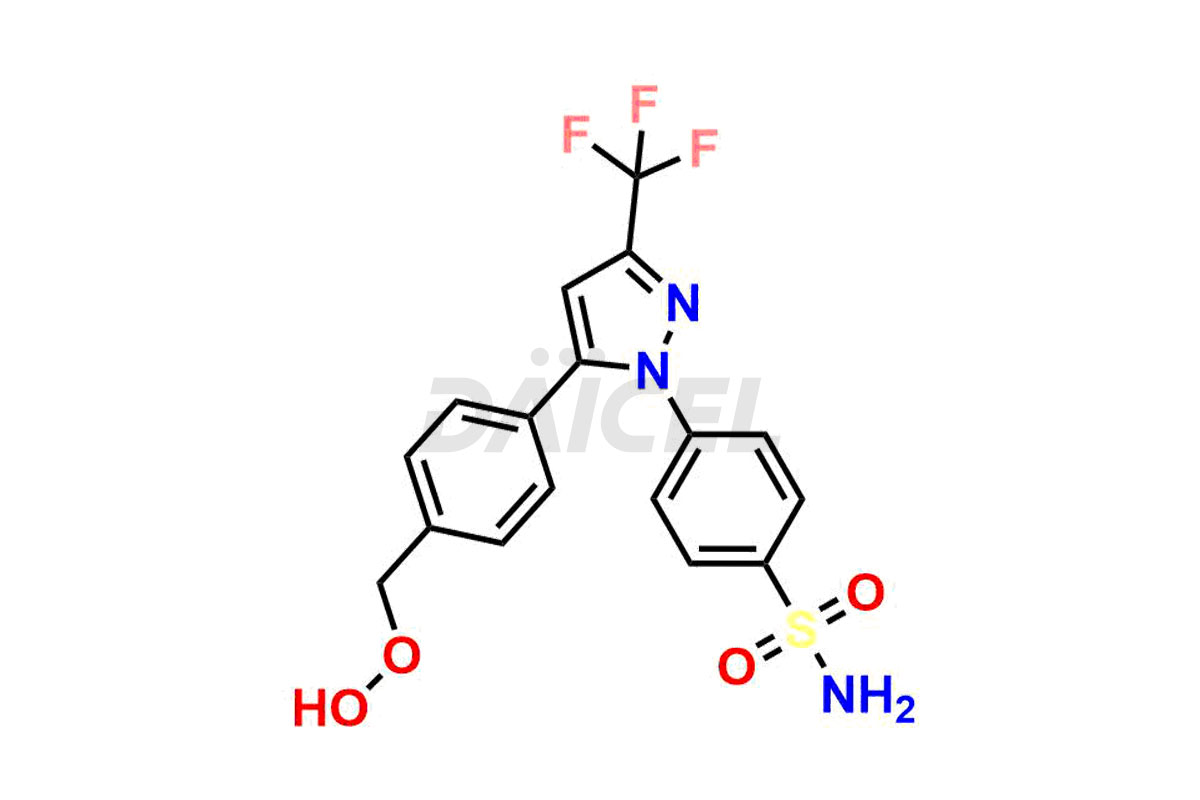
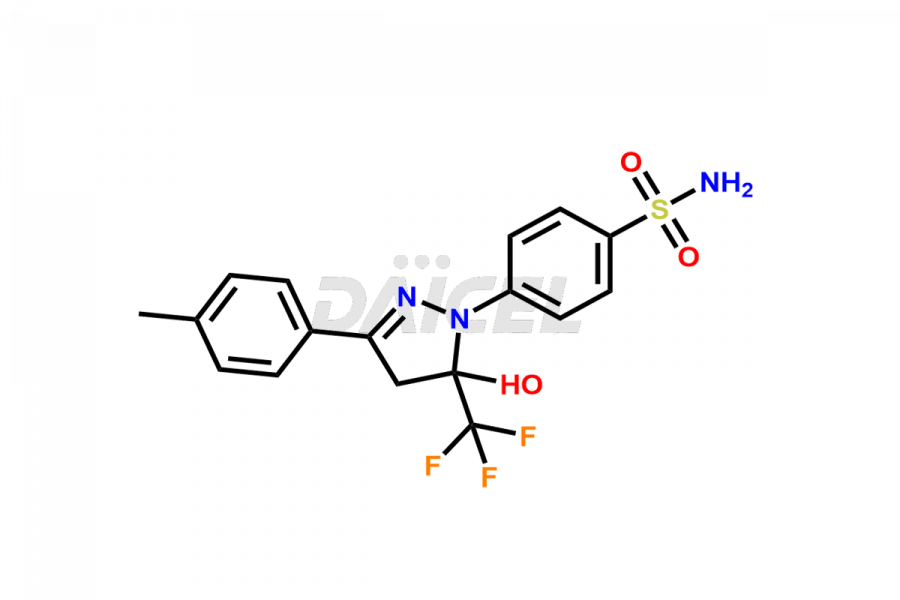
Celecoxib
General Information
Celecoxib Impurities and Celecoxib
Daicel Pharma synthesizes high-quality Celecoxib impurities, 4-(5-(4-(hydroperoxymethyl)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide and Celecoxib Hydroxy impurity, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Celecoxib. Moreover, Daicel Pharma offers custom synthesis of Celecoxib impurities and delivers them globally.
Celecoxib [CAS: 169590-42-5] is a medicine that belongs to the group of nonsteroidal anti-inflammatory drugs (NSAIDs). It is a diaryl-substituted pyrazole.
Celecoxib: Use and Commercial Availability
Celecoxib is a medicine to relieve symptoms in adults with osteoarthritis and rheumatoid arthritis. It treats acute from various sources, like ankylosing spondylitis, juvenile rheumatoid arthritis, and dysmenorrhea. It is available under brand names such as Celebrex, Elyxyb, Consensi, and Seglentis.
Celecoxib Structure and Mechanism of Action 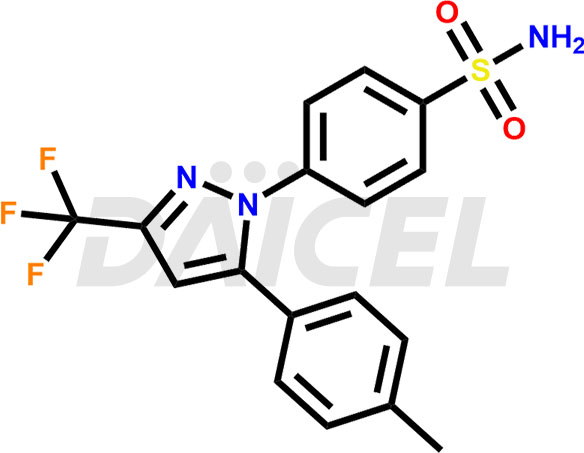
The chemical name of Celecoxib is 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide. Its chemical formula is C17H14F3N3O2S, and its molecular weight is approximately 381.4 g/mol.
Celecoxib has anti-inflammatory and analgesic properties. It is due to the inhibition of prostaglandin synthesis via cyclo-oxygenase-2 (COX-2) inhibition, used in treating arthritis.
Celecoxib Impurities and Synthesis
The impurities formed during the manufacturing1 of Celecoxib include related substances, degradants, and enantiomeric. These impurities form during the synthesis, isolation, purification, and storage of the drug substance. Various factors like reaction conditions, starting materials, and raw materials influence the formation of impurities. Controlling and minimizing the Celecoxib impurities is critical to ensure its quality and safety for patients.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Celecoxib impurity standards, 4-(5-(4-(hydroperoxymethyl) phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide and Celecoxib Hydroxy impurity. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Celecoxib impurity or degradation product.
References
FAQ's
References
- Talley, John J.; Penning, Thomas D.; Collins, Paul W.; Rogier, Donald J., Jr.; Malecha, James W.; Miyashiro, Julie M.; Bertenshaw, Stephen R.; Khanna, Ish K.; Granets, Matthew J., Substituted pyrazolyl benzenesulfonamides, G. D. Searle and Co., United States, US5466823A, NOV 14, 1995
- Rose, M. J.; Woolf, E. J.; Matuszewski, B. K., Determination of celecoxib in human plasma by normal-phase high-performance liquid chromatography with column switching and ultraviolet absorbance detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 738, Issue: 2, Pages: 377-385, 2000
- Srinivasu, M. K.; Narayana, C. L.; Rao, D. S.; Reddy, G. O., A validated LC method for the quantitative determination of celecoxib in pharmaceutical dosage forms and purity evaluation in bulk drugs, Journal of Pharmaceutical and Biomedical Analysis, Volume: 22, Issue: 6, Pages: 949-956, 2000
Frequently Asked Questions
How are Celecoxib impurities controlled during manufacturing?
Impurities in Celecoxib are controlled during manufacturing by using appropriate raw materials, solvents, and reagents, optimizing the synthesis process, and implementing suitable purification and isolation techniques.
What is the role of analytical testing in detecting Celecoxib impurities?
Analytical testing plays a crucial role in detecting impurities in Celecoxib by providing accurate and reliable data on impurity levels to ensure compliance with regulatory guidelines and assess the safety and efficacy of the drug product.
Which solvent is for the analysis of Celecoxib impurities?
Typically, Acetonitrile is used as a solvent for analyzing Celecoxib impurities.
What are the temperature conditions required to store Celecoxib impurities?
Celecoxib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.